Lyon (France), January 17, 2023 – 6 pm CET – SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris ISIN: FR0014005I80 / Ticker: ALSMA, eligible for PEA-PME equity savings plans), a French player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today published its annual sales for the year to December 31, 2022.
Philippe ROUSSOULY, Chairman and CEO of SMAIO, said: “During our first year of listing, SMAIO continued its commercial development with a strong increase in its international sales. To support and boost this expansion, we have set up international educational programs, the first three of which were successfully held in Sweden, Spain and the United States in the second half of 2022. Our objective is to make SMAIO’s technological offer known and available to a maximum of surgeons in countries of strategic interest. Since the IPO, we have also continued our interactions with our partner NuVasive, in order to achieve, in the first half of 2023, a first milestone of 3 million dollars followed by recurring sales by providing imaging analysis services for NuVasive’s clients. We look forward to 2023 with confidence and expect even stronger growth with the ramp-up of new countries, with priority given to the United States, the world’s largest spine market.”
2022 annual sales: strong growth of +60% in international sales
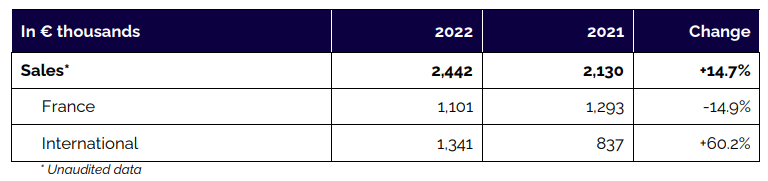
Annual sales totaled €2.4 million in 2022, an increase of 14.7% compared with 2021 (2.1 million euros). Sales of implants and rods accounted for almost all of the Company’s revenue (94%). The software segment, with the Keops platform, accounted for the balance (6%). This segment will grow in coming halves, notably following the granting of FDA’s 510(k) clearance for the Balance Analyzer 3D software and thanks to the partnership and licensing agreement with NuVasive.
International sales jumped 60.7% to 1.3 million euros (55% of total sales). This solid momentum was due to:
- the development of activity with historical distributors in Spain and Scandinavia.
- the signing of new distribution agreements in the Baltic States and Greece;
- the first surgeries performed in the United States. In France, sales declined slightly to approximately 1.1 million euros. Domestic activity should see a return to growth in 2023 thanks to the marketing of the i-Kontrol solution to new medical facilities.
2022 highlights: gradual ramp-up in line with the roadmap
- Partnership with NuVasive, a global leader in spine technology innovation
Within the framework of this partnership, NuVasive has committed to invest a total of $10 million (€9 million1) in the Company, of which $5 million (~€4.5 million) has already been invested at the time of the Company’s IPO in early April 2022, the balance consisting of milestone payments to be made when the Company receives FDA 510(k) clearance for two software solutions interfacing with the US group’s technological platforms.
- Two 510(k) clearances to accelerate development in the United States
In June 2022, SMAIO announced that it had been granted two 510(k) clearances by the FDA for the key components of its i-Kontrol platform: the Balance Analyzer 3D surgical planning software and the custom-made union rod, “K-rod”. With these approvals, SMAIO is now able to offer its i-Kontrol solution to North American centers.
- Success of the “Sagittal Alignment Academy” educational programs
During the second half of 2022, SMAIO successfully held three “Sagittal Alignment Academy” sessions for spine surgeons in Europe (Copenhagen for Northern Europe and Madrid for Southern Europe, in September) and North American (Dallas, Texas, in November). Bringing together several dozen surgeons, these programs aim to accelerate the diffusion of SMAIO’s technology internationally.
Interview with Philippe Roussouly: https://youtu.be/hPZQ8oDWQ_E
Upcoming financial publication
• FY 2022 results: April 12, 2023, after market
About SMAIO
A precursor in the use of clinical data and imaging of the spine, SMAIO designs global solutions for spine surgery specialists. The Company has recognized expertise thanks to KEOPS, its Big Data management software that has become a global reference with more than 100,000 patient cases documented.
SMAIO offers spine surgeons a comprehensive platform, I-Kontrol, incorporating planning, implants and related services, enabling them to treat spinal pathologies in a safe, effective and lasting way.
SMAIO is positioned at the forefront of innovation with the ambition of providing surgeons with the first active robotic solution enabling a high level of performance and repeatability to be achieved.
Based in Lyon, France, SMAIO benefits from the skill and expertise of more than 30 highly specialized staff.
For further information, please visit our website: www.smaio.com
Listing market: Euronext Growth Paris
ISIN: FR0014005I80
Mnemonic: ALSMA
Disclaimer
This press release contains non-factual elements, including, but not limited to, certain statements regarding future results and other future events. These statements are based on the current vision and assumptions of the management of the Company. They incorporate known and unknown risks and uncertainties that could result in significant differences in results, profitability and expected events. In addition, SMAIO, its shareholders and its affiliates, directors, officers, counsels and employees have not verified the accuracy of, and make no representations or warranties about, statistical information or forecast information contained within this news release and that originates or is derived from third party sources or industry publications; these statistical data and forecast information are only used in this press release for information purposes. Finally, this press release may be drafted in French and in English. In the event of differences between the two texts, the French version will prevail.
1 Based on an indicative exchange rate of 1 euro for 1.10 dollars
Contacts
SMAIO
Philippe Roussouly
Chief Executive Officer
Renaut Fritsch
Chief Financial Officer
[email protected]
NewCap
Dusan Oresansky/Quentin Massé
Investor Relations
[email protected]
Tel.: +33 (0)1 44 71 94 92
NewCap
Arthur Rouillé
Media Relations
[email protected]
Tel.: +33 (0)1 44 71 94 98
SOURCE:https://www.smaio-finance.com/images/PR_SMAIO_2022-sales_final.pdf
