SANTA CLARA, Calif., March 06, 2019 (GLOBE NEWSWIRE) — SI-BONE, Inc. (Nasdaq: SIBN), a medical device company that pioneered minimally invasive surgery of the sacroiliac (SI) joint with the iFuse Implant System® (iFuse), announced publication of 2-year results from iMIA (iFuse Implant System Minimally Invasive Arthrodesis; ClinicalTrials.gov ID NCT01741025), a multicenter European RCT in the Journal of Bone and Joint Surgery (JBJS).
iMIA is a Level 1 RCT, conducted at nine hospitals in four countries in Europe, that assessed the safety and effectiveness of SI joint fusion (SIJF), also referred to as SI joint arthrodesis in the manuscript, with the triangular iFuse Implants compared to conservative management (CM) in patients with chronic SI joint dysfunction. The JBJS publication, titled Randomized Trial of Sacroiliac Joint Arthrodesis Compared with Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint,1 showed that minimally invasive SIJF with iFuse Implants was safe and more effective than CM in relieving pain, reducing disability, and improving patient function and quality of life at two years.
“What I find remarkable is the consistency of the results between the 2-year iMIA and 2-year INSITE RCTs,” said David Polly, Jr., MD, Professor, Department of Orthopedic Surgery at the University of Minnesota. “The studies, which were well designed and well executed, were conducted at a combined 28 different centers on two different continents, yet the results are almost identical, which only further validates the effectiveness of the use of the iFuse triangular titanium implants for the treatment of patients with chronic SI joint dysfunction who no longer respond to conservative treatment.”
The iMIA 2-year results were published in JBJS, which has an impact factor of 4.84, the highest among orthopedic journals, and is a highly valued source of information for orthopedic surgeons and researchers. As such, this publication, which is the 67th peer-reviewed iFuse publication, is a significant indication of the broad acceptance of SI joint surgery as an important topic of study in orthopedics.
“The publication of this Level 1 study in a prestigious orthopedic journal such as JBJS is an important milestone signaling recognition of the sacroiliac joint, like all other joints in the human body, as a pain generator and acknowledgement of the high quality of evidence that supports iFuse as an effective minimally invasive procedure for patients with SI joint pain”, said Daniel Cher, MD, Vice President of Clinical Affairs at SI-BONE, Inc.
As shown in Figures 1 and 2 below, 2-year results from iMIA were remarkably consistent with 2-year results from INSITE (Investigation of Sacroiliac Fusion Treatment – NCT01681004), the RCT conducted at 19 institutions in the U.S. and published in August, 2016 in the International Journal of Spine Surgery.2
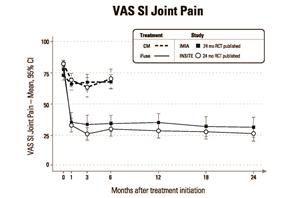
Figure 1 VAS SI Joint Pain
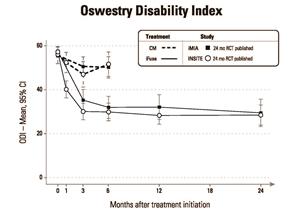
Figure 2 Oswestry Disability Index
In the iMIA study, 103 subjects were randomly assigned to CM (n=51) or SIJF (n=52) between June 6, 2013 and May 15, 2015. At 2 years, mean low back pain (as measured on the Visual Analog Scale, or VAS) improved by 45 points in the SIJF group and 11 points in the CM group (mean difference between groups 34 points, p<0.0001). Mean ODI improved by 26 points in the SIJF group and 8 points in the CM group (mean difference 18 points, p<0.0001). Parallel improvements were seen in leg pain and several quality of life measures. Moreover, objective improvements were observed in SI joint function, including active straight leg raise test and a number of positive physical examination signs for SI joint pain. In the SIJF group, the prevalence of opioid use decreased from 56% at baseline to 33% at 2 years (p<0.01), while no significant change was observed in the CM group (47.1% and 45.7%). Subjects in the CM group who derived minimal benefit after 6 months of CM showed improvements in all measures similar to those originally assigned to SIJF after crossing over to surgery. One case of postoperative nerve impingement occurred in the SIJF group. The full article can be found at the following link: https://journals.lww.com/jbjsjournal/Fulltext/2019/03060/Randomized_Trial_of_Sacroiliac_Joint_Arthrodesis.4.aspx
About SI-BONE
SI-BONE is a medical device company that pioneered the iFuse Implant System, a minimally invasive surgical system for fusion of the sacroiliac joint to treat sacroiliac joint dysfunction. The SI joint is the last major joint with a clinically proven surgical treatment. The iFuse Implant, commercially available since 2009, is the only SI joint fusion device supported by multiple prospective clinical studies showing improved pain, patient function and quality of life resulting from treatment. There are more than 65 peer-reviewed publications supporting the safety, durable effectiveness, and biomechanical and economic benefits unique to the iFuse Implant (www.si-bone.com/results). This body of evidence has enabled multiple government and private insurance payors to establish coverage of the SI joint fusion procedure exclusively when performed with the iFuse Implant System.
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit.
SI-BONE and iFuse Implant System are registered trademarks of SI-BONE, Inc. ©2019 SI-BONE, Inc. All Rights Reserved. 10309.030719
- Dengler J, Kools D, Pflugmacher R, Gasbarrini A, Prestamburgo D, Gaetani P, Cher D, Van Eeckhoven E, Annertz M, Sturesson B. Randomized Trial of Sacroiliac Joint Arthrodesis Compared with Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint. J Bone Joint Surg. 2019;101(5):400-411. doi: 10.2106/JBJS.18.00022
- Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, Cher DJ, Wine KD, Sembrano JN, and the INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016;10:Article 28. doi: 10.14444/3028
