Éragny-sur-Oise, France, January 13, 2020– Safe Orthopaedics (FR0012452746 – ALSAF), a company specializing in the design and marketing of single-use implants and instruments for the minimally invasive treatment of spinal fracture conditions, is today announcing its 2019 fourth quarter turnover, 2019 annual turnover and cash position.
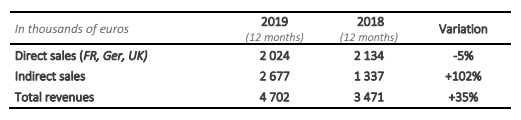
In 2019, Safe Orthopaedics turnover was 4 702 K€, an increase of +35% compared to the year 2018, driven by indirect sales forces and mainly the Japanese partnership. The reduction of annual direct sales by 5% is only recognized on French market. The management had announced in September 27th, 2019, a restructuration and reinforcement of sales and marketing forces, in order to reinvigorate the sales by means of a different commercial approach and the renewal of SteriSpine PS range.
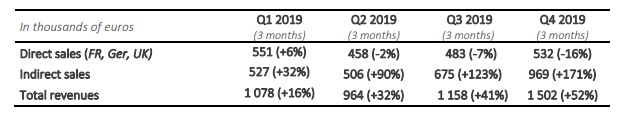
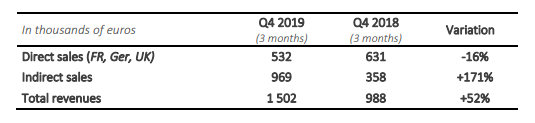
2019 fourth quarter turnover for the company was the best quarterly performance of the year, with a turnover of 1 502 K€, an increase of +52% compared to 2018 fourth quarter turnover. In 2019, the quarterly growth of the company is between +16% and +52%. The management pursues the commercial structuration, necessary for this strategical segment, thanks to the deployment of the next generation of SteriSpine PS, a clear winner with the surgeons (see press release of October 16th , 2019).
In France, direct sales fell slightly and amount to 393k€. The continuing reorganization of the team initiated in 2018, the new generation of SteriSpine PS and the launching of new technologies on the first quarter of 2020 should revive growth. In Germany, annual sales reach 96k€ with a strong growth during the fourth quarter, which sees sales increasing threefold compared to the fourth quarter of 2018. In the United Kingdom, the growth of direct sales during the fourth quarter allowed to ensure a 13% growth compared to the fourth quarter of 2018, with annual sales amounting to 297k€, an increase of 62% that contributes significantly to the gross margin of the company. Indirect sales are growing rapidly thanks to the strategical Japanese partnership and some historical distributors. Sales amount to 969k€ for the fourth quarter, an increase of 171% compared to 2018. The launching of SteriSpine PS at international level should maintain a double-digit growth in 2020.
“2019 was again a constructive year for Safe Orthopaedics, with the reinforcement of its financial resources that cover the cash requirements of the company until 2022, the release of the new generation of SteriSpine PS, the realization of the sales related to the Japanese partnership agreed in 2018 and the change in governance through the nomination of new board members announced on November 26th .” explained Pierre Dumouchel, CEO and co-founder of Safe Orthopaedics. “Through a higher pace of new technologies, a new commercial approach for the 3 countries on direct sales, partnerships on markets with strong potential (Japan, US…), Safe Orthopaedics will maintain growth rates in 2020 at least equivalent to the strong performance of 2019. Finally, the company is today reflecting of innovative solutions for commercialization and production in order to achieve financial equilibrium quicker and reduce the dilution of each shareholder”. Cash position As of today, Safe Orthopaedics’ cash position amounts to 575 k€.
Financial calendar
- Publication of 2019 annual results (URD + PR): April, 28 2020
- Publication of Q2 revenues: July 9, 2020
- Publication of S1 results: September 28, 2020
- Publication of Q3 revenues: October 8, 2020
- Publication of S2 revenues: January 14, 2021
About Safe Orthopaedics
Founded in 2010, Safe Orthopaedics, is a French medical technology company that offers the safest technologies to treat spinal fractures. Delivered sterile, all implants and respective disposable instrumentation are available to the surgeon at any time, anywhere. These technologies enable minimally invasive approaches, redcucing risks of cross contamination and infection in the interest of the patient. Protected by 17 patent families, SteriSpineTM kits are CE marked and FDA cleared. The company is based in Eragny-sur-Oise (95) and has 50 employees. For more information : www.SafeOrthopaedics.com
