Éragny-sur-Oise, Fleurieux-sur-l’Arbresle, April 13th, 2023 at 5:45 p.m. CET – Safe (ALSAF), a company specializing in the design, manufacture and marketing of ready-to-use technologies for back surgery, particularly safe for spinal fractures treated in emergency (the “Company”) announces its first quarter 2023 revenues and cash position.
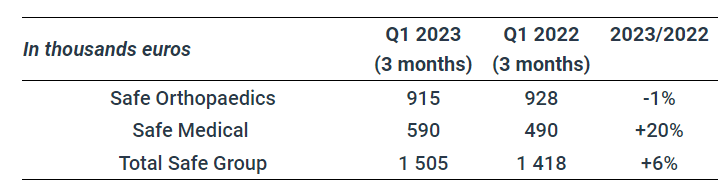
In the first quarter of 2023, the Safe Group’s revenue was €1505k, showing growth of +6% compared with the first quarter of 2022.
Safe Orthopaedics sales were stable at €915k, compared with €928k the previous year.
Direct sales amounted to €594k thanks to strong commercial growth in France (+45%) and the UK (+25%). Indirect sales contracted by 6% to €321k in the first quarter, explained by the still limited post-covid investments by distributors.
Sales of new technologies amounted to €73K in Q1. After their Franco-German evaluation, Sycamore and Hickory are in full commercial roll-out and are awaiting their approvals in the United States as well as their availability on indirect markets. SORA is still being evaluated in France and should be deployed in a German center in the second quarter.
Safe Medical’s business grew by 20% to €590k compared with the first quarter of 2022. This growth is mainly generated by historical customers, who are repositioning Safe Medical as a strategic supplier.
“First-quarter sales amounted to €1.5 million, up thanks to the strong performance of Safe Orthopaedics in France and the United Kingdom and the renewed confidence of Safe Medical’s long-standing customers. The new technologies and the partnership with Wenzel should enable us to boost our commercial execution in Germany and the United States, the largest European and global markets respectively,” commented Pierre Dumouchel, Chairman and CEO and co-founder of Safe Orthopaedics. “Thanks to the new financing agreement announced in March, we have initiated structural changes that should enable us to reduce operating expenses and working capital requirements while maintaining the investments necessary for the commercial growth of our Safe technologies and services.
Cash position
Following a significant refinancing of Safe SA in the amount of nearly €30 million last March, paid in monthly instalments of €600 thousand for the first four instalments and €500 thousand for the following ones, the Group’s unaudited cash position at March 31, 2023 amounted to €268k thousand.
It is reminded that the press release published on March 14, 2023 underlines the risks inherent to this type of financing, which is potentially highly dilutive.
Financial Agenda
| Communication financière | Date1 |
| Annual results 2022 | April 28th 2023 |
| 2023 first semester revenues | 7 juillet 2023 |
| First semester results and Q3 2023 sales | 10 octobre 2023 |
| 2023 annual sales | 10 janvier 2024 |
About Safe Group
Safe Group is a French medical technology group that brings together Safe Orthopaedics, a pioneer in ready-to-use technologies for spine pathologies, and Safe Medical (formerly LCI Medical), a medical device subcontractor for orthopaedic surgery. The group employs approximately 150 people.
Safe Orthopaedics develops and manufactures kits combining sterile implants and single-use instruments, available at any time to the surgeon. These technologies are part of a minimally invasive approach aimed at reducing the risks of contamination and infection, in the interest of the patient and with a positive impact on hospitalization times and costs. Protected by 18 patent families, SteriSpineTM kits are CE marked and FDA approved. Safe Orthopaedics is headquartered in the Paris region (95610 Eragny-sur-Oise) and has subsidiaries in the United Kingdom, Germany, the United States, and the Lyon region (Fleurieux-sur-l’Arbresle).
For more information: http://www.safeorthopaedics.com/
Safe Medical produces implantable medical devices and ready-to-use instruments. It has an innovation center and two production sites in France (Fleurieux-sur-l’Arbresle, 69210) and in Tunisia, offering numerous industrial services: design, industrialization, machining, finishing and sterile packaging. Supported by the French stimulus plan in 2020, the company invests in additive printing and will be operational in 2022 on this new technology.
For more information: http://www.safemedical.fr/
Contacts
Safe Group
François-Henri Reynaud
Chief Financial and Administrative Officer
Tél. : +33 (0)1 34 21 50 00
[email protected]
