Eragny-sur-Oise, France, May 2nd, 2022 08h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces today its 2021 results and reiterates its 2022 strategic objectives.
Safe group’s 2021 Annual Financial Report will be available on the Company’s website (www.SafeOrthopaedics.com) in the Investors > Documentation > Regulated Information section as of April 30, 2022.
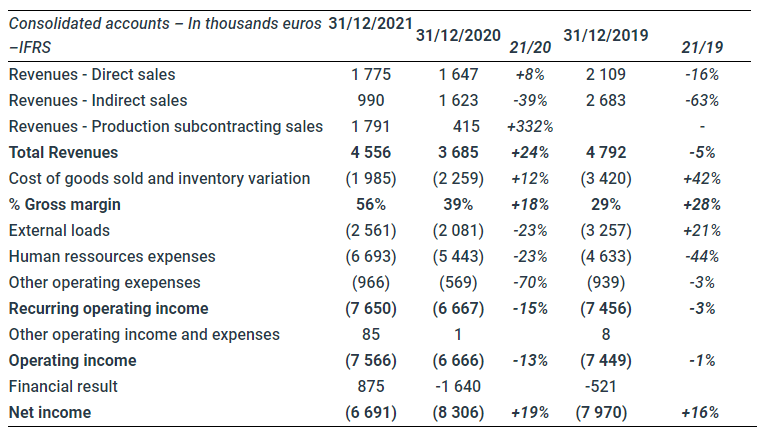
In 2021, revenues reached €4,556k (after the impact of IFRS 15, which deducts sales agent costs directly from revenues), up 23.6%, driven by an increase in direct sales by Safe Orthopaedics and Safe Medical, although the Covid-19 pandemic continues to limit surgical procedures and commercial approaches.
Direct annual sales of Safe Orthopaedics increased by 8% thanks to the performance in Germany (+€263 thousand) and the start of marketing in the USA (+€122 thousand). To enable investments in the latter two territories and improve European operating income, a mutualization of sales and marketing staff and a reduction in the number of French sales teams (5 in 2021 versus 9 in 2020) have been implemented without reducing national coverage.
Safe Orthopaedics indirect sales reached €990 thousand, strongly impacted by the covid over the last two financial years 2020 and 2021. In Japan, the Otsuka Group sold the distribution of Safe Orthopaedics products to Teijin Medical, creating a temporary slowdown.
Safe Medical sales fully integrated into the Group in 2021 amounted to €1.8 million, up 106% between the second half of 2021 and 2020 (the company was acquired in July 2020). In parallel with this commercial acceleration, the construction of an Integrated Innovation and Production Center (CIPI) in Fleurieux-sur-l’Arbresle has been completed, and as of September 2021 will provide all the industrial equipment (microbiological cleaning and clean rooms) necessary for the in-house production of ready-to-use medical devices: the transfer of production of Safe Orthopaedics kits has begun and all SteriSpineTM ranges will be produced there in the first half of 2022.
The group’s first financial synergies are beginning to significantly increase Safe group’s gross margin by 18%. After the transfer of SteriSpineTM technologies in the first half of 2022, the gross margin will be maximized, production lead times will be halved and working capital requirements optimized.
External expenses increased by 23% directly linked to the return to growth of the Group, the launch of new technologies such as Hickory, Sycamore or SORA and investments in Safe Medical, but reduced by €700k compared to 2019 for a similar level of sales. Safe Orthopaedics also accelerated its clinical investments by strengthening its clinical, quality and regulatory affairs staff in order to meet the new European MDR (Medical Device Regulation) requirements and demonstrate the clinical benefits of Sycamore (3-month clinical performance was published on December 20, 2021).
The 23% increase in human resources expenses is explained by the reintegration of Safe Medical’s workforce over a full year (compared to 5 months in 2020), the end of government financial aid during the lockdown period in 2020 and by the recruitment of new clean room operators enabling integrated production and a reduction in external expenses in the last quarter of 2021. As previously explained, an optimization of Safe Orthopaedics’ workforce has been initiated and correlated with commercial expectations in the direct markets (FR, UK, GER and USA) in order to improve the commercial contribution and respect the objective of financial balance within 24 to 36 months.
A review of the 2019 financial statements shows that while the health crisis was still in full swing in 2021, the operating result caught up with the pre-crisis level.
After a positive financial result of €0.87 million made up of currency variations in the subsidiaries’ current accounts, the net income stands at €6.69 million, an improvement of 19.4% compared to the previous year.
“2021 was a year that was rich in creating value: the launch of new differentiating technologies such as Sycamore and SORA, the acceleration of sales in Germany, the commercialization of our technologies in the United States, the industrial and commercial development of Safe Medical. Even though the COVID-19 pandemic is still disturbing the execution, our sales have grown by 24% in 2021, our current operating income has recovered to the 2019 level even though significant investments have been made to build our Safe group, support our innovation and the conversion of the global market to our ready-to-use technologies” comments Pierre Dumouchel, Chairman and CEO of Safe group “Our 2022 roadmap was communicated at the beginning of the year and can be summarized in 4 main axes: Deploying quality global distribution delivering accelerated double-digit growth, innovating and digitizing the surgical act, reducing our ecological impact and improving our financial performance to reach financial balance in 3 years. 2022 starts in accordance with our plan: 31% growth driven by the US and Germany, several dozen SORA and Sycamore surgeries, 80% of our technologies produced in-house.”
Safe reaffirms its strategic objectives for 2022:
- Deploying high-quality global distribution that delivers accelerated double-digit growth. Our experienced sales and marketing teams are focused on driving adoption of our ready-to-use products and promoting their medico-economic benefits to hospitals, purchasing organizations and national health systems.
Sales in the US were €120K in Q1 2022 versus €129K for the full year 2021 (as a reminder, a sales person was recruited in February 2021). Sales outside the US reached €1.3M in Q1 2022 versus €1.07k in Q1 2021. The new Sycamore and Hickory technologies have already boosted sales by €30k while new customers were acquired at the end of the first quarter. The signature of an agreement with Clinicpartner was also published during the quarter.
- Continue to innovate and digitalize the surgical act. Initiated by the S.O.R.A. (Safe Operating Room Assistant) program, our teams are working with surgeons and hospitals worldwide to deploy the surgical act 2.0, offering digital support from the first patient consultation in the operating room through to post-operative clinical follow-up. This data is extremely interesting for the design and production of our technologies.
Several dozen SORA-assisted surgeries have already been performed in the first French evaluation center. The close collaboration with its team of surgeons has made the technology more reliable, initiated new functionalities and accelerated sales by an average of 84% over two quarters. Safe Orthopaedics plans to deploy additional units in France in the second quarter and internationally in the second half of 2022.
- Reducing our environmental impact. In conjunction with the SORA program and the modernization of its plants, the Group is working on the validation of “green-kits”. Safe Orthopaedics is committed to reducing by 30% the waste generated in the operating room, and more globally the consumption of water, energy and CO2 emissions through its CIPI.
Since the qualification of the clean rooms in the summer of 2021, Safe Medical’s French site has been certified IS013485 by the AFAQ on this new industrial perimeter and 80% of Safe Orthopaedics technologies are produced there.
- Aiming for financial break-even in the medium term. Despite the economic and commercial consequences of COVID-19, which has limited the number of spinal surgeries performed in healthcare institutions worldwide, Safe Group continues to deliver double-digit growth and is aiming to break even within three years.
Safe held an investor meeting on April 14th to present its progress and financial strategy. The Group will publish its financial results for the first half of 2022 on 29 September 2022 and has already announced a new investor meeting on 22 September 2022 in Fleurieux sur l’Arbresle.
Cash flow
With a significant refinancing of €8.0 million at the level of Safe SA at the end of the year, and the receipt of a €0.8 million subsidy from the stimulus plan, the balance of which was received in March 2022, the Group’s cash position amounted to €912,000 at December 31, 2021.
About Safe Group
Safe Group is a French medical technology group that brings together Safe Orthopaedics, a pioneer in ready-to-use technologies for spine pathologies, and Safe Medical (formerly LCI Medical), a medical device subcontractor for orthopedic surgery. The group employs approximately 150 people.
Safe Orthopaedics develops and manufactures kits combining sterile implants and single-use instruments, available at any time to the surgeon. These technologies are part of a minimally invasive approach aimed at reducing the risks of contamination and infection, in the interest of the patient and with a positive impact on hospitalization times and costs. Protected by 18 patent families, SteriSpineTM kits are CE marked and FDA approved. Safe Orthopaedics is headquartered in the Paris region (95610 Eragny-sur-Oise) and has subsidiaries in the United Kingdom, Germany, the United States and the Lyon region (Fleurieux-sur-l’Arbresle).
For more information: www.safeorthopaedics.com
Safe Medical produces implantable medical devices and ready-to-use instruments. It has an innovation center and two production sites in France (Fleurieux-sur-l’Arbresle, 69210) and in Tunisia, offering numerous industrial services: design, industrialization, machining, finishing and sterile packaging. Supported by the French stimulus plan in 2020, the company invests in additive printing and will be operational in 2022 on this new technology.
For more information: www.safemedical.fr
Contacts
Safe Group
François-Henri Reynaud
Chief Financial and Administrative Officer
Tél. : +33 (0)1 34 21 50 00
[email protected]
Press Relations
Ulysse Communication
Pierre-Louis Germain / +33 (0)6 64 79 97 51 / [email protected]
Bruno Arabian / +33 (0)6 87 88 47 26 / [email protected]
