Eragny-sur-Oise, France, January 13th, 2022 17h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces its 2021 annual revenue and its cash position.
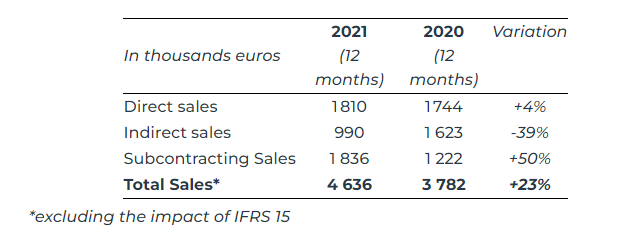
In 2021, the Group’s sales amounted to €4,636 thousand, up 23%, mainly due to the strong growth experienced by Safe medical, up by 50%.
Direct sales by Safe Orthopaedics were up by 4%, with growth increasing quarter after quarter: -18% in Q1, +6% in Q2, +13% in Q3 and +19% in Q4 driven by accelerating sales in Germany (€429k, 160%) and the start of marketing in the US (€103k). France experienced a 15% decline over the year and saw a return to growth of +4% in the last quarter.
Internationally, the global health situation is still having a strong impact on indirect sales, given the multiple local confinements observed.
Safe medical’s sales growth, up 50% compared to 2020, validates the group’s model, which was created in 2020 and is based on an industrial production tool with high added value for its customers. It should be noted that the packaging infrastructure has only been operational since the end of 2021. It will constitute a new growth vector in 2022 by providing an additional service to Safe medical’s customers while shortening their production lead times, as will the additive printing planned for the second half of 2022.

“The structuring of the Safe Group is a winning strategy, which, thanks to the plurality of revenue sources and commercial territories, offers double-digit growth in a global context still disrupted by the health crisis. The commercial performance of Safe Medical and Safe Orthopaedics’ direct sales give us confidence for 2022,” commented Pierre Dumouchel, Chairman and CEO of Safe Group and co-founder of Safe Orthopaedics. “2021 will also have been a year of innovation and investment to structure our 2022 growth through the qualification of Safe Medical, the launch of Sycamore (estimated global market of more than one billion dollars), Hickory and SORA, marketed worldwide in 2022. The combination of our two entities, Safe Orthopaedics and Safe Medical, will enable us to maintain the pace of innovation and consolidate our position as a pioneer and leader in orthopaedic ready-to-use products. This new way of managing surgery is now followed by several dozen players, confirming the conversion trend of the global market and is the subject of multiple medico-economic publications”.
The cash position at 31/12/2021 was €0.9M (unaudited figure). The balance of the stimulus plan subsidy still to be received by Safe medical in Q1 2022 is €400k. As a reminder, Safe Group has secured €8m of financing in December 2021.
About Safe Group
Safe Group is a French medical technology group that brings together Safe Orthopaedics, a pioneer in ready-to-use technologies for spine pathologies, and Safe Medical (formerly LCI Medical), a medical device subcontractor for orthopaedic surgery. The group employs approximately 150 people.
Safe Orthopaedics develops and manufactures kits combining sterile implants and single-use instruments, available at any time to the surgeon. These technologies are part of a minimally invasive approach aimed at reducing the risks of contamination and infection, in the interest of the patient and with a positive impact on hospitalization times and costs. Protected by 18 patent families, SteriSpineTM kits are CE marked and FDA approved. Safe Orthopaedics is headquartered in the Paris region (95610 Eragny-sur-Oise) and has subsidiaries in the United Kingdom, Germany, the United States and the Lyon region (Fleurieux-sur-l’Arbresle).
For more information: www.safeorthopaedics.com
Safe Medical produces implantable medical devices and ready-to-use instruments. It has an innovation center and two production sites in France (Fleurieux-sur-l’Arbresle, 69210) and in Tunisia, offering numerous industrial services: design, industrialization, machining, finishing and sterile packaging. Supported by the French stimulus plan in 2020, the companýinvests in additive printing and will be operational in 2022 on this new technology.
For more information: www.safemedical.fr
Contacts
Safe Group
François-Henri Reynaud
Chief Financial and Administrative Officer
Tél. : +33 (0)1 34 21 50 00
[email protected]
Press Relations
Ulysse Communication
Pierre-Louis Germain / +33 (0)6 64 79 97 51 / [email protected]
Bruno Arabian / +33 (0)6 87 88 47 26 / [email protected]
