LEWISVILLE, Texas–(BUSINESS WIRE)–Orthofix Medical Inc. (NASDAQ:OFIX), a leading global medical technology company, today reported its financial results for the second quarter ended June 30, 2025, and reaffirmed its full-year 2025 financial guidance. All pro forma measures contained within this release exclude the impact of the Company’s decision to discontinue its M6® product lines.
Highlights
- Second quarter 2025 net sales of $203.1 million, including sales from M6® artificial cervical and lumbar discs, and pro forma net sales of $200.7 million, excluding sales from M6® discs, representing an increase of 2% on a reported basis and 4% on a pro forma constant currency basis compared to second quarter 2024
- U.S. Spine Fixation1 net sales growth of 5% and procedure volume growth of 7% compared to second quarter 2024
- Bone Growth Therapies (“BGT”) net sales of $62.6 million, representing growth of 6%, with BGT Fracture net sales growth of 7% compared to second quarter 2024
- Global Orthopedics net sales of $33.3 million, achieving constant currency growth of 5%, and U.S. Orthopedics net sales growth of 28% compared to second quarter 2024
- Initiated global commercial launch of the TrueLok® Elevate Transverse Bone Transport (“TBT”) System – the first FDA-cleared device for TBT to correct non-unions and bony or soft tissue deformities or defects
- Announced U.S. commercial launch of the Reef® L Interbody System – completes Reef® interbody product family with a full-spectrum solution for lateral lumbar spinal fusion procedures
- Second quarter 2025 net loss of $(14.1) million on a reported basis; Non-GAAP pro forma adjusted EBITDA of $20.6 million, with pro forma adjusted EBITDA margin expanding approximately 190 basis points compared to reported non-GAAP adjusted EBITDA for the second quarter 2024
- Six consecutive quarters of adjusted EBITDA margin expansion; Positive free cash flow of $4.5 million for second quarter 2025
Second quarter 2025 net sales were $203.1 million, including sales from M6® artificial cervical and lumbar discs, and pro forma net sales of $200.7 million, excluding sales from M6® discs, representing an increase of 2.3% on a reported basis and 3.5% on a pro forma constant currency basis compared to second quarter 2024. Net loss was $(14.1) million, or $(0.36) per share, on a reported basis. Non-GAAP pro forma adjusted EBITDA was $20.6 million for the second quarter of 2025, an increase of $4.0 million compared to reported non-GAAP adjusted EBITDA of $16.6 million for the second quarter of 2024, representing 24.1% growth over the prior year.
“Orthofix’s second quarter results demonstrate clear progress on our three-year plan to transform the business,” said Massimo Calafiore, President and Chief Executive Officer. “Our disciplined approach led to strong adjusted EBITDA margin growth and positive free cash flow generation, underscoring our ability to grow the business responsibly. Strategic initiatives, like accelerating distributor transitions in certain underpenetrated U.S. territories, are gaining traction and creating a powerful foundation for a more scalable commercial organization to drive our next phase of growth. Looking ahead, we expect to benefit from our recent product launches and deliver meaningful product innovation to improve outcomes and efficiencies for our surgeons and their patients. I am confident the Company is well positioned to deliver sustainable, long-term shareholder value throughout the second half of 2025 and beyond.”
1 Spine Fixation is comprised of the Company’s Spinal Implants product category, excluding motion preservation product offerings.
Financial Results Overview
Second Quarter 2025 Net Sales and Financial Results
The following table provides net sales by major product category and by reporting segment on a pro forma basis, removing the effects of the Company’s discontinued M6® product lines:
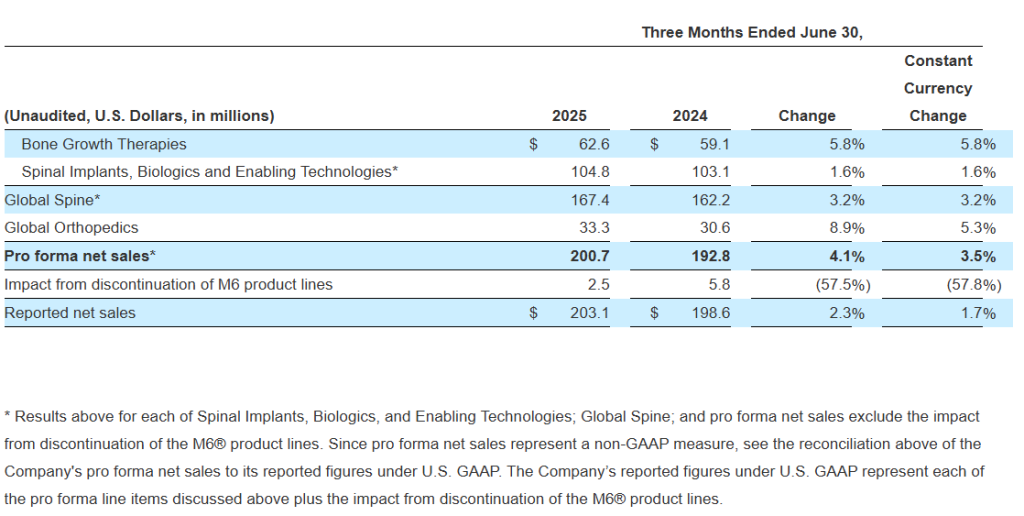
Gross margins were 68.7% for the quarter and were 72.7% on a non-GAAP pro forma adjusted basis.
Net loss was $(14.1) million, or $(0.36) per share, on a reported basis, compared to net loss of $(33.4) million, or $(0.88) per share in the prior year period. Non-GAAP pro forma adjusted EBITDA was $20.6 million, or 10.3% of pro forma net sales, compared to reported non-GAAP adjusted EBITDA of $16.6 million, or 8.4% of reported net sales, in the prior year period.
Liquidity
Cash, cash equivalents, and restricted cash on June 30, 2025 totaled $68.7 million compared to $60.5 million on March 31, 2025.
Business Outlook
The Company is reaffirming its full-year net sales guidance and its full-year 2025 adjusted EBITDA and free cash flow guidance as follows:
- Pro forma net sales expected to range between $808 million to $816 million, excluding sales from the discontinued M6® product lines. This guidance range is based on current foreign currency exchange rates and does not take into account any additional potential exchange rate changes that may occur this year.
- Pro forma non-GAAP adjusted EBITDA is expected to be $82 million to $86 million. This range includes the anticipated impact from the discontinuation of the M6® product lines that was previously announced in February 2025.
- Free cash flow is expected to be positive for full-year 2025, excluding the impact of restructuring charges related to the discontinuation of the M6® product lines.
An investor presentation for the Company’s second quarter 2025 financial results is available in the “Events & Presentations” section of the Orthofix Investor Relations Website at ir.orthofix.com.
Conference Call
Orthofix will host a conference call today at 8:30 AM Eastern time to discuss the Company’s financial results for the quarter ended June 30, 2025. Interested parties may access the conference call by dialing (888) 596-4144 in the U.S., and (646) 968-2525 in all other locations, and referencing the conference ID 4830464. A webcast and replay of the conference call may be accessed in the “Events & Presentations” section of the Orthofix Investor Relations Website at ir.orthofix.com.
Internet Posting of Information
Orthofix regularly shares important updates in the “Investors” section of its website at www.orthofix.com. The Company encourages investors and potential investors to consult the Orthofix website regularly for important information about Orthofix.
About Orthofix
Orthofix is a global medical technology company headquartered in Lewisville, Texas. By providing medical technologies that heal musculoskeletal pathologies, Orthofix delivers exceptional experiences and life-changing solutions to patients around the world. Orthofix offers a comprehensive portfolio of spinal hardware, bone growth therapies, specialized orthopedic solutions, biologics and enabling technologies, including the 7D FLASH™ Navigation System. To learn more, visit Orthofix.com and follow on LinkedIn.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, intentions, plans, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” “positioned,” “deliver,” or “continue” or other comparable terminology. Forward-looking statements in this communication include the Company’s expectations regarding net sales, adjusted EBITDA, and free cash flow for the year ended December 31, 2025. Forward-looking statements are not guarantees of our future performance, are based on our current expectations and assumptions regarding our business, the economy and other future conditions, and are subject to risks, uncertainties and changes in circumstances that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2024, and in Part II, Item 1A under the heading Risk Factors in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2025. Factors that could cause future results to differ from those expressed by forward-looking statements include, but are not limited to, (i) our ability to maintain operations to support our customers and patients in the near-term and to capitalize on future growth opportunities, (ii) risks associated with acceptance of surgical products and procedures by surgeons and hospitals, (iii) development and acceptance of new products or product enhancements, (iv) clinical and statistical verification of the benefits achieved via the use of our products, (v) our ability to adequately manage inventory, (vi) our ability to successfully optimize our commercial channels, (vii) our success in defending legal proceedings brought against us, and (viii) the other risks and uncertainties more fully described in our periodic filings with the Securities and Exchange Commission (the “SEC”). As a result of these various risks, our actual outcomes and results may differ materially from those expressed in these forward-looking statements.
Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. The Company undertakes no obligation to update, and expressly disclaim any duty to update, its forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise, except as required by law.
The Company is unable to provide expectations of GAAP net income (loss), the closest comparable GAAP measures to adjusted EBITDA (which is a non-GAAP measure), on a forward-looking basis because the Company is unable to predict, without unreasonable efforts, the ultimate outcome of matters (including acquisition-related expenses, accounting fair value adjustments, and other such items) that will determine the quantitative amount of the items excluded in calculating adjusted EBITDA, which items are further described in the reconciliation tables and related descriptions below. These items are uncertain, depend on various factors, and could be material to the Company’s results computed in accordance with GAAP.
