LEWISVILLE, Texas–(BUSINESS WIRE)– Orthofix Medical Inc. (NASDAQ:OFIX) today reported its financial results for the quarter ended March 31, 2023. Net sales were $175.2 million, earnings per share (“EPS”) was $(1.71), and adjusted EPS was $(0.10).
“Orthofix delivered an exceptionally strong quarter with 12% year-over-year growth on a proforma basis and by minimizing any disruption associated with the business combination in January,” said Keith Valentine, President and Chief Executive Officer of Orthofix. “We are encouraged by the meaningful pro forma growth we’ve seen across all channels in the combined company and remain enthusiastic about the opportunities afforded by our complementary portfolios. Our team was successful in leveraging cross-selling strategies throughout the quarter and we believe these initiatives will promote meaningful market share gains in the future.”
Financial Results Overview
The following table provides net sales by major product category by reporting segment as reported:
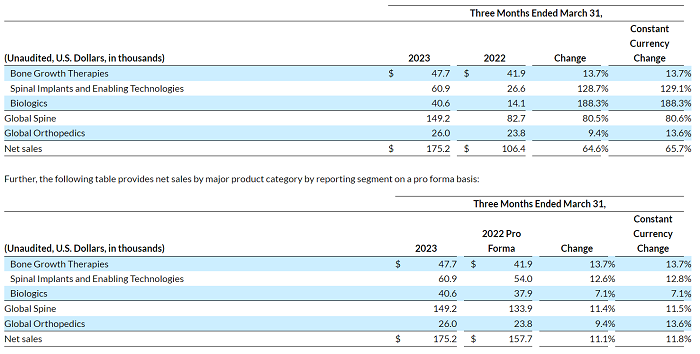
Gross profit increased $32.2 million to $110.3 million. Gross margin decreased to 63.0% compared to 73.4% in the prior year period. Adjusted gross profit increased $45.6 million to $123.9 million. Adjusted gross margin decreased to 70.7% compared to 73.6% in the prior year period.
Net loss was $(60.9) million, or $(1.71) per share, compared to net loss of $(4.5) million, or $(0.22) per share in the prior year period. Adjusted net loss was $(3.6) million, or $(0.10) per share, compared to adjusted net income of $1.0 million, or $0.05 per share, in the prior year period.
Adjusted EBITDA was $3.2 million, or 1.8% of net sales, compared to $7.1 million, or 6.7% of net sales, in the prior year period.
Liquidity
As of March 31, 2023, cash totaled $50.0 million, compared to $50.7 million as of December 31, 2022. As of March 31, 2023, the Company had $45.0 million in borrowings outstanding under its five year $300 million secured revolving credit facility. For the first three months of 2023, cash flow from operations decreased $26.3 million to $(34.0) million, while free cash flow decreased $32.5 million to $(45.9) million.
Business Outlook
As of the date hereof, the Company expects the following net sales results for the year ended December 31, 2023. These expectations are based on the current foreign currency exchange rates and do not include any additional exchange rate changes that may occur this year.
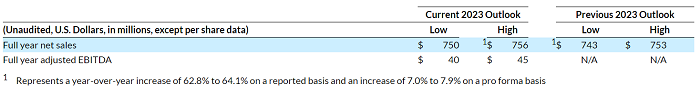
The Company is unable to provide expectations of GAAP operating income (loss), the closest comparable GAAP measures to Adjusted EBITDA (which is a non-GAAP measure), on a forward-looking basis because the Company is unable to predict without unreasonable efforts the ultimate outcome of matters (including acquisition-related expenses, accounting fair value adjustments, and other such items) that will determine the quantitative amount of the items excluded in calculating Adjusted EBITDA, which items are further described in the reconciliation tables and related descriptions below. These items are uncertain, depend on various factors, and could be material to the Company’s results computed in accordance with GAAP.
Conference Call
Orthofix will host a conference call today at 4:30 PM Eastern time to discuss the Company’s financial results for the first quarter of 2023. Interested parties may access the conference call by dialing (888) 330-2508 in the U.S. and Canada, and (240) 789-2735 in all other locations, and referencing the access code 9556380. A replay of the call will be available for three weeks by dialing (800) 770-2030 in the U.S. and Canada, and (647) 362-9199 in all other locations, and entering the access code 9556380. A webcast of the conference call may be accessed at ir.Orthofix.com.
About Orthofix
The newly merged Orthofix-SeaSpine organization is a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions and a leading surgical navigation system. Its products are distributed in approximately 68 countries worldwide. The Company intends to announce a new name for the Orthofix-SeaSpine organization in the future, but in the interim will continue to operate under the Orthofix name.
The Company is headquartered in Lewisville, Texas, and has primary offices in Carlsbad, CA, with a focus on spinal product innovation and surgeon education, and Verona, Italy, with an emphasis on product innovation, production, and medical education for Orthopedics. The Orthofix-SeaSpine organization’s global R&D, commercial and manufacturing footprint also includes facilities and offices in Irvine, CA, Toronto, Canada, Sunnyvale, CA, Wayne, PA, Olive Branch, MS, Maidenhead, UK, Munich, Germany, Paris, France, and Sao Paolo, Brazil. For more information, please visit www.orthofix.com.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. Forward-looking statements in this communication include the Company’s belief regarding future market share gains and the Company’s expectations regarding net sales and adjusted EBITDA for the year ended December 31, 2023. Forward-looking statements are not guarantees of our future performance, are based on our current expectations and assumptions regarding our business, the economy and other future conditions, and are subject to risks, uncertainties and changes in circumstances that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2022 (the “2022 Form 10-K”), and in Part II, Item 1A under the heading Risk Factors in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023. Factors that could cause future results to differ from those expressed by forward-looking statements include, but are not limited to, (i) risks relating to the effects of the COVID-19 pandemic on our business, (ii) our ability to maintain operations to support our customers and patients in the near-term and to capitalize on future growth opportunities, (iii) risks associated with acceptance of surgical products and procedures by surgeons and hospitals, (iv) development and acceptance of new products or product enhancements, (v) clinical and statistical verification of the benefits achieved via the use of our products, (vi) our ability to adequately manage inventory, (vii) our ability to recruit and retain management and key personnel, (viii) global economic instability and potential supply chain disruption caused by Russia’s invasion of Ukraine and resulting sanctions, and (ix) the other risks and uncertainties more fully described in our periodic filings with the Securities and Exchange Commission (the “SEC”). To the extent that the COVID-19 pandemic continues to adversely affect our business and financial results, it may also have the effect of heightening many of the other risks described in Part I, Item 1A under the heading Risk Factors in the 2022 Form 10-K, such as our ability to generate sufficient cash flows to run our business and our ability to protect our information technology networks and infrastructure from unauthorized access, misuse, malware, phishing and other events that could have a security impact as a result of our remote working environment or otherwise. As a result of these various risks, our actual outcomes and results may differ materially from those expressed in these forward-looking statements.
This list of risks, uncertainties, and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the SEC, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.
SOURCE: Orthofix Medical
