NeuroStructures is excited and pleased to announce the FDA 510k approval of the Transept™ Cervical Plating System which features the ability to achieve extreme screw angulation (up to 38°) and extremely short plates (10mm).
The plate has an ultra-thin profile and comes with a wide spectrum of fixed, variable, self-tapping, and self-drilling screws. Also included, are the new unified screws (variable or fixed self-drilling), which work particularly well in ‘poor bone’. Instruments are user friendly and fully customizable incorporating multi-use instrumentation focused on streamlining the surgical process.
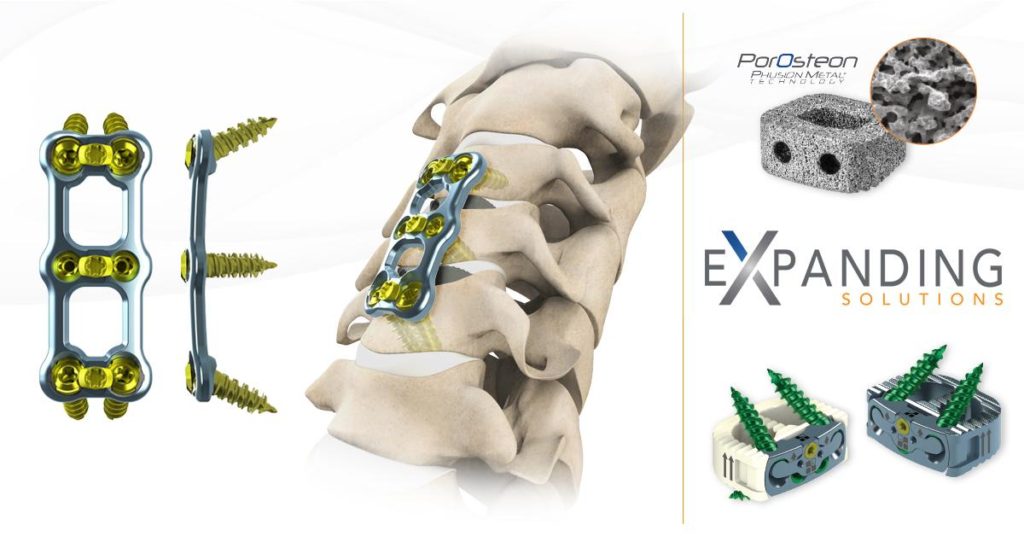
Advanced Osteoconductive Technology
With material stiffness within the range of cancellous bone and an irregular porous structure with fully interconnected passageways — macro, micro, and nano sized pores — the Cavetto® Phusion Metal® facilitates bone integration, thus, actively participates during the entire fusion process.
What´s in the Works?
Want to know what we have currently in our robust R & D pipeline? Follow us to keep up to date with our newest product offerings such as Oculus™-SA Lumbar Cage System and our expanding solutions technology products such as PLIF, TLIF, Lateral Lumbar Cages, Stand Alone Cervical Cage, and Stand Alone ALIF Cage.
About NeuroStructures
NeuroStructures is an employee-owned and operated medical device company focused on designing, developing, manufacturing and marketing proprietary, high-quality medical device systems. All of our products provide comprehensive medical solutions to improve and enhance the quality of life for patients. NeuroStructures is dedicated to exceeding expectations in product quality, customer service, and product cost. The company is led by a team of experienced marketing, engineering and sales individuals with extensive knowledge and training in the domestic and international spine surgery device markets. http://www.neurostructures.com
