Lyon and New York, July 9, 2020 – The MEDICREA® Group (Euronext Growth Paris: FR0004178572 –ALMED ; OTCQX Best Market –MRNTF), pioneering the transformation of spinal surgery through Artificial
Intelligence, predictive modeling and patient specific implants with its UNiD™ ASI (Adaptive Spine Intelligence) proprietary software platform, services and technologies, publishes sales for the first half of 2020.
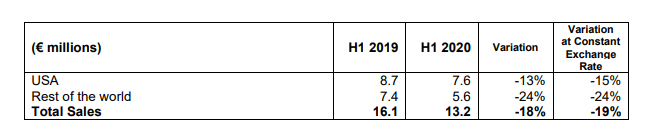
Sales for the 2nd quarter amounted to €5.8 million, bringing the half-year total to €13.2 million, down 18% compared to the first half of 2019 due to the COVID-19 pandemic. The postponement of many elective surgeries since mid-March had a strong negative impact on activity over this period.
As soon as the containment measures and the mobilization of healthcare institutions in many countries were announced, MEDICREA® prepared for the worst-case scenario, which was planned to have no billings for the second quarter. Despite a very sharp drop in sales in April and May, representing the equivalent of (-25%) and (-66%) of a normative activity, respectively, June sales recovered strongly, totaling €3.2 million, up +3% compared to the same period last year, i.e. a sequential growth of +75% between May 2020 and June 2020.
In June, the Group’s three main markets performed well given the health context:
– In the United States, sales were up by +13% compared to June 2019, despite the continued strong spread of COVID-19 in many States;
– In Belgium, growth was +12% over the same period;
– Sales in France are gradually returning to normal, with some regions having regained good momentum while those most affected by the pandemic are still under limited activity.
The use of UNiD™ patient-specific rods planning and manufacturing services also shows a strong recovery with 144 UNiD® personalized surgeries having taken place in the United States in June, an increase of +33% compared to the same month of the previous year, and 5,850 surgeries performed cumulatively as of June 30, 2020 since the launch of this technology.
« We had planned for a difficult second quarter, but strategic management of the crisis along with the favorable evolution of the COVID-19 pandemic enabled us to resume business faster than expected while generating very strong activity across our various markets. Therefore, our sales recorded a clear rebound as of May 15. The strong sales generated in June 2020 for MEDICREA® demonstrated a positive sign to the potential end of this crisis. The current evolution of the health situation in the United States and in the southern hemisphere obliges us, to remain cautious as the catch-up effect that will inevitably occur is still anticipated for the second half of 2020 but could be delayed by a few months. » commented Denys Sournac, Founder, President and CEO of MEDICREA®.
On June 30, 2020, the cash position was €13.2 million.
The strategic discussions which had resumed in May after a period of interruption due to the spread of the COVID-19 epidemic, are continuing to be very active.
Next publication: 2020 Half-Year results: September 14, 2020 after market
About MEDICREA® (www.medicrea.com)
Through the lens of predictive medicine, MEDICREA® leverages its proprietary software analysis tools with big data and machine learning technologies supported by an expansive collection of clinical and scientific data. The Company is well-placed to streamline the efficiency of spinal care, reduce procedural complications and limit time spent in the operating room.
Operating in a $10 billion marketplace, MEDICREA® is a Small and Medium sized Enterprise (SME) with 175 employees worldwide, which includes 35 who are based in the U.S. The Company has an ultra-modern manufacturing facility in Lyon, France housing the development and production of 3D-printed titanium patient-specific implants.
For further information, please visit: medicrea.com.
SOURCE: https://www.medicrea.com/wp-content/uploads/2020-07-08-Medicrea-HY1-Sales-EN-1.pdf
