August 17, 2023 — Incline Village, Nev., — Kleiner Device Labs today announced that its new KG®2 Surge®flow-thru interbody system was recently used inits 50th surgical case.
Noted founder and CEO Jeff Kleiner, MD, “The new KG2 Surge was developed with the objectives of maximizing bone graft delivery to the prepared intervertebral disc space, and streamlining implant placement, positioning, and integration in the graft matrix. The first fifty cases demonstrated excellent achievement of those objectives. We will next be moving to broader commercial availability, expanding our network of distributors and independent representatives.”
“Overall, I think surgeons have lost track of the importance of meticulous disc space preparation and abundant, quality bone graft delivery and packing into the disc space well enough to achieve high fusion rates,” said surgeon Jeffrey Dick, MD, of Twin Cities Orthopedics. “One of the best things about the KG2 is the ease with which one can deliver abundant bone graft through the tube and how tightly you can pack it compared to conventional techniques.”
Neurosurgeon Catherine Gallo, MD of Two Rivers Surgical Center in Eugene, Oregon,said, “I can tell that the KG2 is a very well-thought-out cage design. Somebody put some real thought into this. It is very user-friendly.”
flow-thru interbody system, which allows implant placement, positioning, and grafting in a
single surgical pass in TLIF and PLIF procedures.
Orthopedic spine surgeon Blake Burkert, MD, of NeuroSpine in Eugene, Oregon, commented, “I will change my approach and do more PLIFs because the KG2 system is so easy to use and the graft volume is impressive. Love this cage system.”
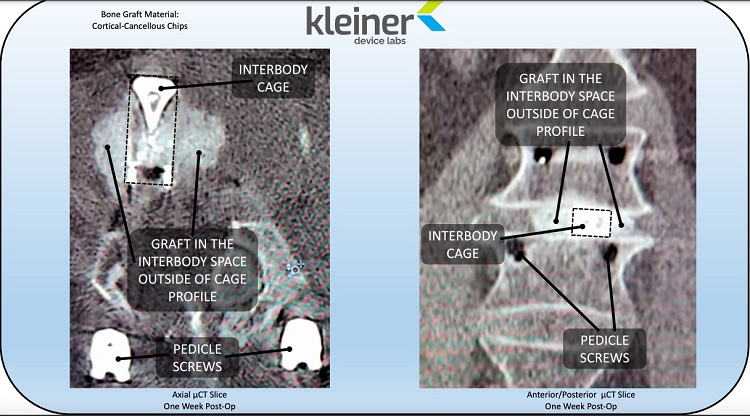
The KG2 Surge flow-thru interbody system is a single-patient-use bone graft delivery tool coupled with a 3D-printed titanium I-Beam fusion implant. The implant has no lateral walls and serves as a conduit for unimpeded flow of a broad spectrum of bone graft materials through the pre-attached, rectangular insertion tool. The rectangular cannula maximizes the cross-sectional area available for graft material flow and eliminates the challenge of trying to apply bone graft after cage insertion. Since the system comes pre-assembled and sterilized in a single use tray, there is minimal scrub tech training and no implant tray re-processing/sterilization — an ideal system for an ASC or hospital. The KG2 system allows for a single insertion process to take the place of the multi-step, multi-instrument pass practice. The surgical procedure with KG2 Surge spares contusion of delicate nerve tissue and reduces the risk of surgical site infection.
For KG2 videos and information, please go to the company’s website.
About Kleiner Device Labs
Kleiner Device Labs is creating new instruments and devices to advance minimally invasive spine surgery and improve outcomes and costs. Kleiner Device Labs is headquartered in Incline Village, Nevada.
KG and Surge aretrademarks of Kleiner Device Labs.
For Kleiner Device Labs:
Sales and Surgeon Contact
Bob Haugen
M: 224.223.2118
MediaContact
Brad Samson
M: 714.955.3951
Investor Contact
Stewart Peabody
M: 312.498.5892
MKT-00010-65 Rev 1
