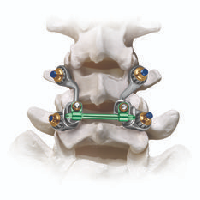
An artificial joint replacement is a prosthesis intended to replace the natural facets and other posterior elements of the spine, restoring near-normal motion while providing stabilization of spinal segments.Its indications are as an adjunct to laminectomy, laminotomy, neural decompression, and facetectomy. It is used instead of a standard lumbar fusion.
Joints (Facet) replacement patents started in the ’80s. However, artificial facet devices appeared in the mid-2000s. In these 20 years, they have had limited progress. Many of them as ACADIA (Globus Medical), TFAS or Zyre (Spinal Elements), do not exist today.
What Joint replacement systems are the most relevant on the market today?
There are currently two lumbar joint replacement systems on the market. TOPS is a facet joint replacement system for the lumbar spine. MOTUS is a total joint replacement for the low back.
1.- TOPS™ System (Premia Spine)
The first and only facet joint replacement system for the lumbar spine, TOPS was developed to provide mobility, stability and durability after decompression for patients with lumbar spinal stenosis and degenerative spondylolisthesis. TOPS won Breakthrough Device Designation from the US Food and Drug Administration (FDA) in 2021.

The TOPS™ System replaces anatomical structures, such as the lamina or the facet joint that are removed from the vertebrae during the spinal decompression treatment to alleviate pain. The internal metal stoppers replace the natural bony elements that served as stoppers during axial rotation. The boot and internal components take the place of the supraspinous ligament, interspinous ligament, and ligamentum flavum in their ability to help control flexion. The TOPS System is anchored to the spine with four standard polyaxial pedicle screws and is implanted via a traditional posterior surgical approach. The TOPS patented crossbar configuration exerts less force on the screws than fusion implant devices. At the same time, TOPS withstands sheer forces that interspinous and intralaminar devices cannot contain.
The TOPS™ System uniquely recreates motion in all directions – flexion, extension, lateral bending, and axial rotation. Clinically proven to provide immediate and sustained pain relief and improvement in quality of life, Premia Spine’s TOPS™ System has also been demonstrated to provide excellent outcomes for appropriate patients. The TOPS System provides three major benefits for the treatment of spinal stenosis. First, the procedure stabilizes the posterior spine and reestablishes a controlled range of movement. Second, patients regain their ability to bend, flex, walk, and enjoy the normal activities of life. Third, and most importantly, patients experience immediate and sustained pain relief. Clinical studies conducted since 2005 show the TOPS System alleviates persistent leg and low back pain for patients with moderate to severe spinal stenosis, degenerative spondylolisthesis (a slipped disc) and facet arthrosis (bone spurs).
- US Regulatory Status: CAUTION – Investigational device. Limited by US law to investigational use.
About Premia Spine
Premia Spine, a medical technology company, aims to improve the lives of chronic leg and back pain patients with its TOPS™ System. TOPS is designed to provide lasting mobility, stability and durability to patients with lumbar spinal stenosis, degenerative spondylolisthesis and related spinal conditions.
Please for more information about the TOPS system visit:https://premiaspine.com/topstm-system/
2.-MOTUS (3Spine)
MOTUS is a total joint replacement for the low back that provides the following benefits:
- Restore Natural Balance
- Maintain Range of Motion
- Preserve Adjacent Spinal Levels
3Spine is built on some basic concepts: safely cross three columns of the spine (posterior, middle, and anterior) thereby gaining access, support, and correction; thereby replacing the function of three structures (two facets and the disc); thereby dynamically balancing the three curves of the spine; thereby transitioning safely between the three sagittal activities of daily living (standing, sitting, and slumped sitting).
- MOTUS Total Joint Replacement VIDEO ANIMATION
- A-novel-total-joint-replacement-may-be-an-improvement-.pdf
- Master Class: Lumbar SPINE Total Joint Replacement
- US Regulatory Status: CAUTION – Investigational device. Limited by US law to investigational use.
About 3Spine
3Spine is a Medtronic spin out. Medtronic purchased three-column IP in 2004 as the foundation for a posterior TJR IP portfolio, subsequently developing the KENAI device. In 2014 the portfolio was spun out to a private shell with Medtronic’s support to complete additional refinements and the US IDE, leveraging private capital. International Surgical purchased the IP in 2016, rebranding as BalancedBack and then MOTUS. International Surgical SEZC became 3Spine SEZC then 3Spine, Inc. 3Spine is now fully capitalized and entirely independent.Please for more information about the MOTUS system visit: https://www.3spine.com
***
NOTE: Article Image by kjpargeter on Freepik
