Bordeaux, Boston, January 24, 2023 – 6:00 pm CET: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral implants, today announced its 2022 annual revenue and its cash position at December 31, 2022.
Ludovic Lastennet, IMPLANET’s Chief Executive Officer, stated: “We ended 2022 with a strong overseas performance. Despite the slowdown we are currently experiencing on the French market, we recorded a solid financial year marked by constant growth. This performance demonstrates the strength of the various partnerships we have deployed abroad and confirms the strategic orientation we have adopted over the last two years. 2023 will be marked by the implementation of our partnership with Sanyou Medical. JAZZ® platform registration has begun in China, and we hope to be able to record the first sales of the ultrasound surgical scalpel developed by Sanyou Medical subsidiary SMTP during the first quarter of 2023. These factors give us confidence that we can achieve our targets of breaking even financially in the medium term and becoming a key player in the treatment of spine disorders, both in France and internationally”.
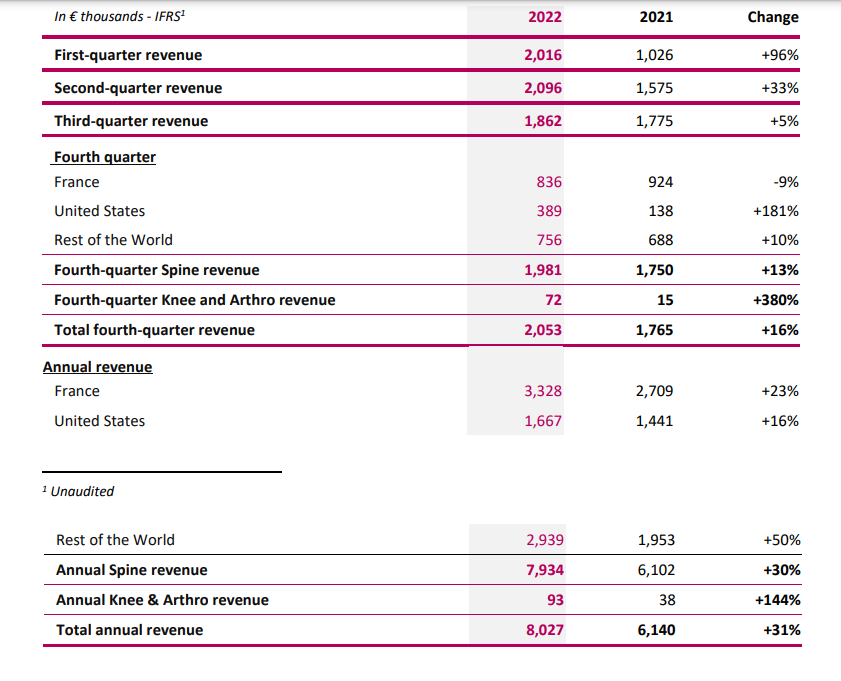
Fourth quarter of 2022
In the fourth quarter of 2022, following three consecutive quarters of growth, activity further increased by 16% to €2.05 million, from €1.77 million in fourth quarter 2021.
Despite activity in France still being hindered notably by hospital staff shortage issues, posted growth was driven by:
- 181% jump in United States revenue, from €0.14 million in fourth quarter 2021 to €0.39 million in the same quarter of 2022;
- acceleration in commercial development in the Rest of the World, with 10% growth in the fourth quarter of 2022 to €0.76 million. Export activity recorded continuous growth through all four quarters of the year.
2022 annual revenue
Over 2022 as a whole, activity increased by 31%, with revenue totaling €8.03 million vs. €6.14 million in 2021. This performance was due to both the organic growth in JAZZ activity (+31% in unit terms and +24% in value terms) and the effect of the OSD acquisition, which represented €2.89 million over the entire 2022 fiscal year compared with €2.04 million from the date of the acquisition in May 2021.
In France, activity grew by 23% from €2.71 million in 2021 to €3.33 million in 2022.
In the United States, activity increased by 16% thanks to the good performances of +17% observed in the third quarter and +181% in the fourth quarter. Revenue generated in the United States thus totaled €1.67 million in 2022 compared with €1.44 million in 2021.
In the Rest of the World, export activity was multiplied by 1.5 (+50%), with revenue rising from €1.95 million in 2021 to €2.94 million in 2022.
Cash position
At December 31, 2022, Implanet had a cash position of €0.5 million. As mentioned in the press release announcing the Company’s third-quarter revenue, Sanyou Medical has informed the Company of its irrevocable intention to exercise the warrants it holds for an amount of €2.5 million during the first quarter of 2023. Furthermore, the payment of the balance of the MADISONTM business, totaling €2.3 million, is spread over time depending on the achievement of regulatory milestones related to CE marking, with €1.5 million expected to be received during the first half of 2023.
These funds will be allocated to the commercial development of the JAZZ® range in China, the finalization of the comprehensive new range of ORION hybrid spinal fixation initiated with Sanyou Medical and the commercial deployment of the ultrasound surgical scalpel developed by SMTP Technology Co, a Sanyou Medical subsidiary.
Reminder of key 2022 highlights
- First surgeries in the United States with JAZZ™ PF, an innovative solution from the JAZZ® line.
- First surgeries in the United States with the ORIGIN Cervical Spine Plate, marking the first successful synergies with OSD products.
- Signing of a commercial, technological and financial partnership with Sanyou Medical, the second largest Chinese manufacturer of medical devices for spine surgery:
- distribution agreement for Implanet’s JAZZ® platform in China, the world’s largest spine surgery market by volume;
- technological partnership: joint development of a brand-new European range of hybrid spinal fixation systems;
- financial partnership: capital increase with preferential subscription rights through the issuance of shares with warrants attached (‘ABSA’), guaranteed for a total amount of €5.0 million by the partner Sanyou Medical.
- Success of the capital increase for a total amount of €2.77 million, including €2.5 million subscribed by Sanyou Medical.
- Finalization of the distribution agreement in China for Implanet’s JAZZ® platform and a technological partnership to develop a new product range.
- Signing of an exclusive distribution contract for France regarding SMTP Technology Co.’s ultrasound surgical scalpel.
Upcoming financial press release
- 2022 annual results, on March 7, after market close
About IMPLANET
Founded in 2007, IMPLANET is a medical technology company that manufactures high-quality implants for orthopedic surgery and distributing medical technology equipment. Its activity revolves around a comprehensive innovative solution for improving the treatment of spinal pathologies (JAZZ®) complemented by the product range offered by Orthopaedic & Spine Development (OSD), acquired in May 2021 (thoraco-lumbar screws, cages and cervical plates). Implanet’s tried-and-tested orthopedic platform is based on the traceability of its products. Protected by four families of international patents, JAZZ® has obtained 510(k) regulatory clearance from the Food and Drug Administration (FDA) in the United States, the CE mark in Europe and ANVISA approval in Brazil. In 2022, IMPLANET entered into a commercial, technological and financial partnership with SANYOU MEDICAL, China’s second largest medical device manufacturer. IMPLANET employs 39 staff and recorded a consolidated revenue of €6.1 million in 2021. Based near Bordeaux in France, IMPLANET opened a US subsidiary in Boston in 2013. IMPLANET is listed on the Euronext Growth market in Paris. For further information, please visit www.Implanet.com.
Contacts
IMPLANET
Ludovic Lastennet, CEO
David Dieumegard, CFO
Tél. : +33 (0)5 57 99 55 55
[email protected]
NewCap
Investor Relations
Mathilde Bohin
Nicolas Fossiez
Tél.: +33 (0)1 44 71 94 94
[email protected]
NewCap
Media Relations
Arthur Rouillé
Tél.: +33 (0)1 44 71 94 94
[email protected]
