GENEVA, SWITZERLAND (PRWEB) JUNE 02, 2016–Eden Spine (http://www.EdenSpine.com), an R&D driven medical technology company, announced today that the SPHYNX™ plating system was granted CE Mark approval.
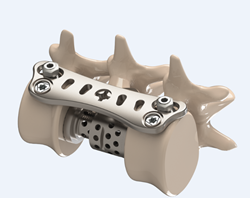
Made of titanium, the low profile SPHYNX™ is to be implanted via the antero-lateral approach for the treatment of thoraco-lumbar instabilities. Indications include spinal fractures, vertebral tumors, secondary instabilities of the thoracic and thoraco-lumbar spine, and any other indication requiring an anterior stabilization low profile.
“The SPHYNX™ is the latest innovation coming from our R&D department in Geneva, Switzerland,” says Mourad Ben Mokhtar, President of Eden Spine Europe, SA. “Our goal was to develop a technology that was simple to use, intuitive, and worked with the anatomy. To achieve that goal we have developed a cutting edge integrated locking system, minimized the thickness of the implant in an effort to respect the surrounding tissues, and maximized the range of precurved plates, to provide optimal adaptation to patient’s anatomy.”
The SPHYNX™ is the ideal complement to Eden Spine proprietary corpectomy device, the GIZA™; an expandable titanium vertebral body replacement implant, with rotatable endplates, that provide multiple angulation options by simple endplates rotation. The GIZA™ is intended to replace and fuse a collapsed, damaged, or unstable vertebral body due to a tumor or a fracture.
Distribution opportunities for the SPHYNX™ and the GIZA™ are available.
About Eden Spine:
Eden Spine LLC is a privately held, technology driven spinal organization headquartered in Florida, with a wholly owned subsidiary in Geneva, Switzerland. The company distributes a range of innovative spinal technologies in the United States, Latin America, Europe, Asia and the Middle East. Eden Spine patented portfolio is composed of a mix of fusion and non-fusion technologies.
