ConnectSPINE ™ PPM ™ is a Transforaminal lumbar Interbody Fusion cage (TLIF) manufactured in Porous Paw Metal for better bone ingrowth with high porosity as compared to cancellous bone.
Features: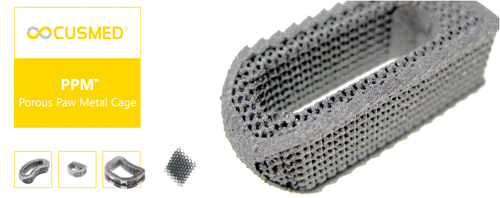
- Ultra-high strength as compared to cortical bone and high porosity structure as compared to cancellous bone.
- Autoclavable – withstands repeated heat sterilization.
- 3D anotomical shape implant availadble may be in “All in 1 Piece” .
- Porous Paw Metal (PPM™): Titanium by PAWTEX™
About TLIF
A transforaminal lumbar interbody fusion (TLIF) is performed to remove a portion of a disc that is the source of back or leg pain. Bone graft is used to fuse the spinal vertebrae after the disc is removed. TLIF provides fusion of the front and back of the lumbar spine. The front portion of the spine, called the anterior column, is stabilized by the interbody spacer and bone graft. The back portion, or posterior column, is locked in place with pedicle screws, rods and additional bone graft, alongside the backs of the vertebra.
CUSMED® focus on innovative medical implants and instruments design and clearance. CUSMED® is rich of integrated manufacturing service and medical image processing to develop CMF, Spine, Trauma and Dental implants even patient specific implant like bone mesh, bone plate, surgical guide and anotomical model. http://www.cusmed.com
