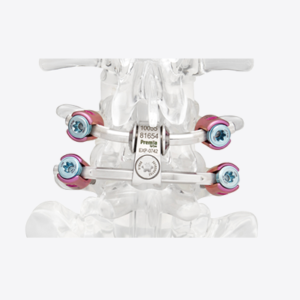
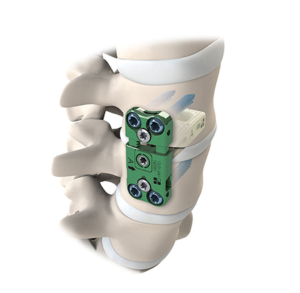
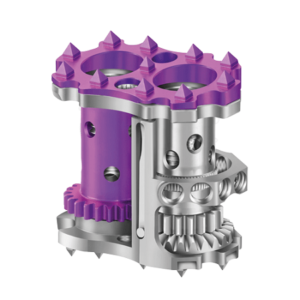
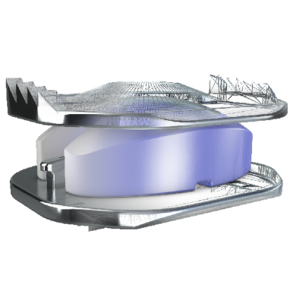
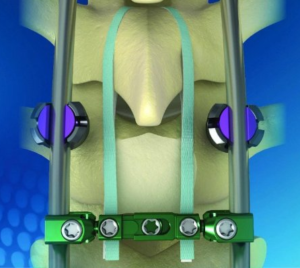
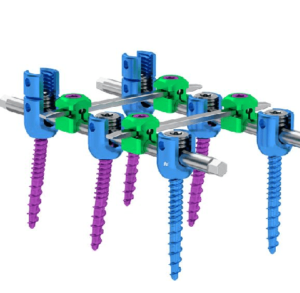
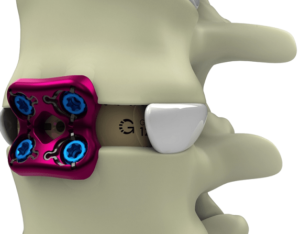
Expanding on the biomechanical and design principles of X-CORE,® the X-CORE 2 Expandable VBR is designed to be the latest and most advanced all-approach expandable VBR on the market. The X-CORE 2 Expandable VBR provides expanded versatility that enables surgeons to utilize X-CORE technology – not only via the XLIF ® approach, but also through […]
NEWS
XRL® Vertebral Body Replacement Device
The XRL® Vertebral Body Replacement Device provides surgeons the ability to expand the device in situ to reconstruct the anterior column, restore height, and correct the sagittal curvature of the thoracolumbar spine. XRL.Brochure-DepuySynthes.pdf XRL.SGT-DepuySynthes.pdf Features: Integrated and modular construction 360º implantation Tactile feedback during expansion Most implant options to accomodate a wide range of anatomies PEEK implant: Ability […]
XPand®
XPand® is an expandable corpectomy device available in a wide variety of footprints, heights and lordotic/kyphotic angles to match varying patient anatomy. Insertion and continuous adjustable expansion is achieved with one specially designed instrument aimed to simplify the surgical technique. Xpand.Brochure-Globus-Medical.pdf Benefits: Non-ratcheting expansion: Allows continuous adjustability within a constrained surgical space Simplified technique: One-step insertion and expansion […]
Xpendriv Cage
Auxein Spine’s Xpendriv Cage Vertebral Body Replacement System is intended to replace a vertebral body or an entire vertebra. It is for use in the thoracolumbar spine (T1-L5) to replace a collapsed, damaged or unstable vertebral body or vertebra resected or excised during total and partial corpectomy and vertebrectomy procedures due to tumor or trauma (i.e., fracture). […]
Fuse Medical, Inc. Completes Acquisition of Maxim Surgical
RICHARDSON, Texas–(BUSINESS WIRE)–Fuse Medical, Inc. (OTC: FZMD) (“Fuse” or the “Company”), announced the completion of the acquisition of Palm Springs Partners, LLC d/b/a Maxim Surgical (“Maxim”), a manufacturing company in the spinal fusion device market and full-service medical device and distribution company (the “Acquisition”). The effective date of Acquisition was August 1, 2018. Formed in […]
10 Spine Companies to Know with “P”
The Spine market is dominated by the following 10 global companies:Medtronic, DePuy Synthes, NuVasive Inc, Globus Medical, Stryker Corporation, Zimmer Biomet, K2M Group Holdings Inc, Alphatec Spine and Aesculap Implant Systems But in addition to these companies, there are more than 300 manufacturers worldwide. Many weeks ago, we started to display the updated list of all […]
Alphatec Reports Second Quarter 2018 Financial Results
CARLSBAD, Calif., Aug. 02, 2018 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a provider of innovative spine surgery solutions with a mission to improve patient lives through the relentless pursuit of superior outcomes, today reported financial results for the second quarter ended June 30, 2018. Second Quarter 2018 Financial Highlights […]
Newly Published Data Demonstrate Bone In-Growth Potential of Stryker’s 3D-Printed Tritanium® Cage
ALLENDALE, N.J.–(BUSINESS WIRE)–Stryker’s Spine division today announced the publication of a pre-clinical animal study comparing the performance of spinal implants made from a variety of materials, which illustrated the bone in-growth and biological fixation capabilities of its 3D-printed Tritanium cages. The study was published in the July issue of The Spine Journal. The purpose of […]
RTI Surgical® and Aziyo® Biologics Announce Exclusive Agreement to Distribute ViBone® for Spine and Other Orthopedic Procedures
ALACHUA, Fla. & SILVER SPRING, Md.–(BUSINESS WIRE)–RTI Surgical, Inc. (Nasdaq: RTIX), a global surgical implant company, and Aziyo Biologics, Inc., a fully integrated regenerative medicine company, today announced the signing of an agreement under which Aziyo will provide ViBone to RTI Surgical for exclusive distribution in the U.S. ViBone is a bone repair product designed to […]
Globus Medical Reports Second Quarter 2018 Results
AUDUBON, Pa., Aug. 01, 2018 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, today announced its financial results for the second quarter ended June 30, 2018. Worldwide sales were $173.4 million, an increase of 13.8% as reported Second quarter net income was $45.0 million, an increase of 56.9% Diluted earnings per share […]
NuVasive Announces Second Quarter 2018 Financial Results
SAN DIEGO, July 31, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced financial results for the quarter ended June 30, 2018. Second Quarter 2018 Highlights Revenue increased 8.5% to $281.6 million, or 7.7% on a constant currency basis; GAAP operating profit margin of 10.1%; […]
OrthoPediatrics, Osseus, and many others…! SPINE Catalogue From A to Z: 18 Spine Companies to know with “O”
The Spine market is dominated by the following 10 global companies:Medtronic, DePuy Synthes, NuVasive Inc, Globus Medical, Stryker Corporation, Zimmer Biomet, K2M Group Holdings Inc, Alphatec Spine and Aesculap Implant Systems But in addition to these companies, there are more than 300 manufacturers worldwide. Many weeks ago, we started to display the updated list of all […]
Nuvasive, Neurostructures, and many others…! SPINE Catalogue From A to Z: 13 Spine Companies to know with “N”
The Spine market is dominated by the following 10 global companies:Medtronic, DePuy Synthes, NuVasive Inc, Globus Medical, Stryker Corporation, Zimmer Biomet, K2M Group Holdings Inc, Alphatec Spine and Aesculap Implant Systems But in addition to these companies, there are more than 300 manufacturers worldwide. Many weeks ago, we started to display the updated list of all […]
Mahe Medical USA launches Spine Emergency Screw Removal system and makes it available via Amazon
Mahe Medical USA announced the introduction and availability of its universal spine emergency screw removal system. “Anything that can be done in the OR to help the patient through a procedure in a shorter period of time without impacting the outcome should be celebrated and that’s exactly what the Mahe spine emergency screw removal system […]
Medtronic, Medacta, Medicon, Medicrea and many others…! SPINE Catalogue From A to Z: 20 Spine Companies to know with “M”
The Spine market is dominated by the following 10 global companies:Medtronic, DePuy Synthes, NuVasive Inc, Globus Medical, Stryker Corporation, Zimmer Biomet, K2M Group Holdings Inc, Alphatec Spine and Aesculap Implant Systems But in addition to these companies, there are more than 300 manufacturers worldwide. Many weeks ago, we started to display the updated list of all […]
SPINE Companies Catalogue From A to Z: 7 Spine Companies to know with “L”
We are already building (week by week) the new SPINE Catalogue that will include most of the Spinal companies worldwide. We want to promote and build awareness of most of the market players, even the smallest ones. Today we present all the Spinal companies which name start with L including LDR, Life Spine and many […]
Global spine market growth 2018-2023. Updated: 150 Companies from “A to K”
Over the recent years, global spinal fusion market has been witnessing growth, on account of several driving factors including rising healthcare awareness amongst consumers, surging prevalence of spinal deformities due to accidents along with increasing health expenditure in developing countries. Moreover, ongoing demographic shift towards geriatric population with significant population suffering from inveterate spinal ailments, […]
SPINE Companies Catalogue From A to Z: 6 Spine Companies to know with “J”
This week, we present the TENTH chapter (J) that includes all the Spinal companies which name start with J including Joimax among others.In that list you can find 6 Companies. Please visit the following page: https://thespinemarketgroup.com/category/spine-companies/page/12/ For any question please contact us: [email protected] or [email protected]
SPINE Companies Catalogue From A to Z: 17 Spine Companies to know with “I”
This week, we present the NINTH chapter (I) that includes all the Spinal companies which name start with I including Innovasis or Implanet among others.In that list you can find 17 Companies. Please visit the following pages: https://thespinemarketgroup.com/category/spine-companies/page/10/ https://thespinemarketgroup.com/category/spine-companies/page/11/ For any question please contact us: [email protected] or [email protected]
We are proud and happy to announce that Rudischhauser Surgical Instruments and Implants Manufacturing is Sponsor of SPINEMarketGroup in 2018!
Thank you Rudischhauser Surgical Instruments and Implants Manufacturing! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About Rudischhauser Surgical Instruments and Implants Manufacturing Your partner and OE-Manufacturer of superior instruments, implants and complete set configurations for spinal, orthopaedic and trauma injuries.Rudischhauser […]
SPINE Companies Catalogue From A to Z: 6 Spine Companies to know with “H”
This week, we present the EIGHT chapter (H) that includes all the Spinal companies which name start with H including HD Lifesciences among others.In that list you can find 6 Companies. Please visit the following page: https://thespinemarketgroup.com/category/spine-companies/page/10/ For any question please contact us: [email protected] or [email protected]
SPINE Companies Catalogue From A to Z: 13 Spine Companies to know with “G”
This week, we present the SEVENTH chapter (G) that includes all the Spinal companies which name start with G including Globus Medical among others.In that list you can find 13 Companies. Please visit the following page: https://thespinemarketgroup.com/category/spine-companies/page/9/ For any question please contact us: [email protected] or [email protected]
Renovis Surgical Receives FDA Clearance for Tesera Trabecular Technology™ Hyperlordotic ALIF Interbody Spinal Fusion System
REDLANDS, Calif. and AUSTIN, Texas, June 28, 2018 /PRNewswire/ — Renovis Surgical Technologies, Inc. today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the Tesera® SA Hyperlordotic ALIF Interbody Spinal Fusion System. Tesera SA is a porous titanium stand-alone anterior lumbar interbody fusion system featuring a […]
EOS imaging Announces First Private Practice Installation of An EOS® System in Germany
PARIS–EOS imaging (Paris:EOSI) (Euronext, FR0011191766 – EOSI – Eligible PEA – PME), the pioneer of 2D/3D imaging and data solutions for orthopedics, today announced the installation of an EOS® imaging system at ATOS Klinik Heidelberg, establishing it as first private practice in Germany to offer the low-dose 2D/3D imaging system. The system will be available at […]
GS Medical Announces New Product Launch and Successful Cadaver Workshop
IRVINE, Calif., June 27, 2018 (GLOBE NEWSWIRE) — GS Solutions Inc., DBA GS Medical USA (GSM), a leader and innovator of surgeon-driven spinal technology that advances patient care, today announced the launch of the AnyPlus® Extended Tab Minimally Invasive Pedicle Screw system. The AnyPlus® Extended Tab Minimally Invasive Pedicle Screw System was engineered to offer […]
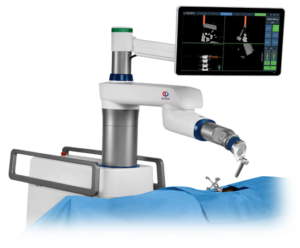
Globus Medical Announces ExcelsiusGPS® Milestone: 3,000 screws implanted
AUDUBON, Pa., June 26, 2018 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, announced today a new clinical milestone with the successful implantation of over 3,000 screws using ExcelsiusGPS®, the company’s revolutionary robotic guidance and navigation system. Since the launch of ExcelsiusGPS® in late 2017, orthopedic and neurosurgeons have used the […]
Spineart announces the first European PERLA® surgeries
Spineart is proud to announce the first European PERLA® surgeries that simultaneously took place in Spain with Dr. ULLOA and Dr. DARDER, in Ireland with Dr. POYNTON and in Germany with Prof. RINGEL. PERLA® is a Posterior Cervical System offering a complete range of sterile-packed implants and intuitive instruments in a single compact set.The system […]