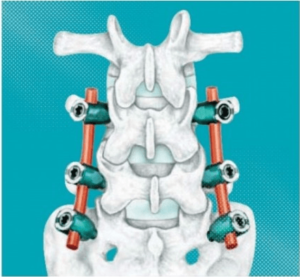
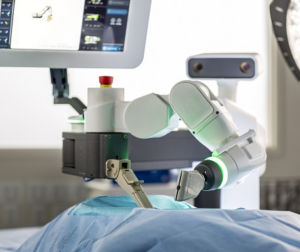
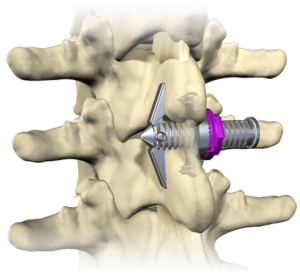
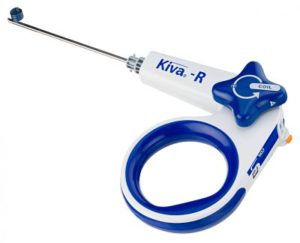
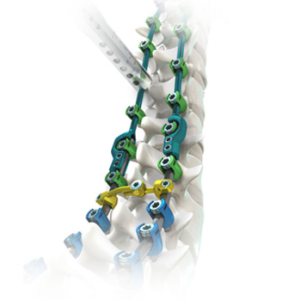
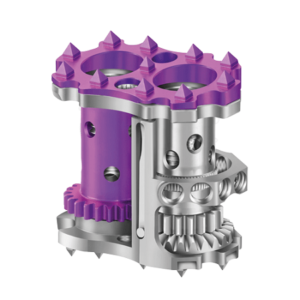
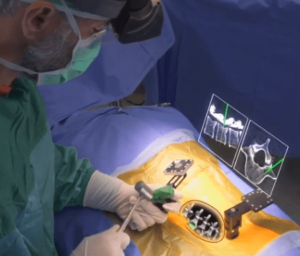
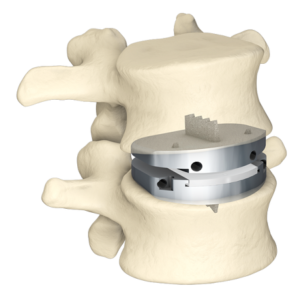
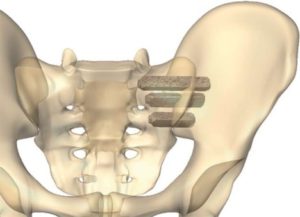
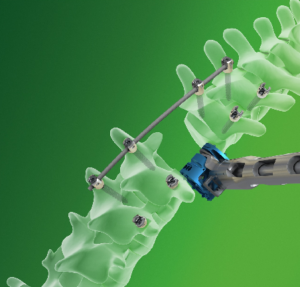
SAN DIEGO and DONAUESCHINGEN, GERMANY, Oct. 9, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced it has entered into a strategic partnership with Biedermann Technologies GmbH & Co. KG, a company that holds a broad and extensive patent and technology portfolio in the […]
NEWS
60 Spine Companies to Know with “S”
Over the recent years, global spinal fusion market has been witnessing growth, on account of several driving factors including rising healthcare awareness amongst consumers, surging prevalence of spinal deformities due to accidents along with increasing health expenditure in developing countries. Moreover, ongoing demographic shift towards geriatric population with significant population suffering from inveterate spinal ailments, […]
Amedica Announces Closing of the SALE of the Sale of its Spine Business to CTL Medical
SALT LAKE CITY, Oct. 03, 2018 (GLOBE NEWSWIRE) — Amedica Corporation (NASDAQ: AMDA) today announced that it has completed the previously-announced sale of its spine business to CTL Medical, a Dallas, TX-based privately held medical device manufacturer, on October 1, 2018, all as more fully described below. As previously announced, Amedica and CTL Medical entered […]
AI And Robotics Are Taking Robotic Surgery To New Levels
(INVESTORS.COM)–With artificial intelligence now firmly entrenched in many hospital operating rooms, the field of robotic surgery is starting to get competitive.Giant companies like Alphabet (GOOGL), Johnson & Johnson (JNJ) and Medtronic(MDT) are training their sights on Intuitive Surgical (ISRG), the king of robotic surgery companies. But analysts say the booming medical technology segment has lots of room to run. Robotic surgery is […]
Medtronic – Adding Some Robotics To The Mix
(SEEKINGALPHA.COM)– (MDT) has announced a rather interesting bolt-on deal, although the timing of the deal can be questioned. The company is acquiring robotic guidance system provider Mazor Robotics (MZOR) in a $1.64 billion deal, as the cash component comes in $300 million less thanks to net cash holdings of Mazor, and the fact that Medtronic already holds a […]
10 MORE Spine Companies to Know with “S”
The Spine market is dominated by the following 10 global companies:Medtronic, DePuy Synthes, NuVasive Inc, Globus Medical, Stryker Corporation, Zimmer Biomet, K2M Group Holdings Inc, Alphatec Spine and Aesculap Implant Systems.But in addition to these companies, there are more than 300 manufacturers worldwide. Many weeks ago, we started to display the updated list of all the […]
Medtronic to Acquire Mazor Robotics
DUBLIN and CAESAREA, Israel – September 20, 2018 – Medtronic plc (NYSE:MDT), a global leader in medical technology, and Mazor Robotics (NASDAQ:MZOR, TASE:MZOR.TZ), a pioneer in the field of robotic guidance systems, today announced the companies have entered into a definitive merger agreement under which Medtronic will acquire all outstanding ordinary shares of Mazor for […]
Zavation Medical Products, LLC, a LongueVue Capital Portfolio Company, Completes Investment in Pan Medical U.S. Corp.
JACKSON, Miss., Sept. 18, 2018 /PRNewswire/ — Zavation Medical Products (“Zavation”), a LongueVue Capital (“LVC”) portfolio company, is pleased to announce it has partnered with the management team of Pan Medical U.S. Corp (“PanMed” or the “Company”) to acquire the Company. This partnership broadens Zavation’s service offering by adding a full suite of minimally invasive products, including the […]
Globus Medical Acquires Surgimap®
AUDUBON, Pa., Sept. 13, 2018 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, today announced the acquisition of Nemaris Inc., a privately held company that markets and develops Surgimap®, a leading surgical planning software platform. Surgimap® allows healthcare professionals to simulate potential surgical outcomes and share medical imaging globally to improve […]
IZI Medical Products Acquires Benvenue Medical’s Vertebral Augmentation Systems
OWINGS MILLS, Md.–(BUSINESS WIRE)–IZI Medical Products, LLC, a leading interventional medical device company, announced it has completed the acquisition of Benvenue Medical, Inc.’s vertebral augmentation systems. The acquisition includes the Kiva®, Kiva® Pilot, and Blazer® devices in addition to other needles, injectors and cements. This acquisition complements IZI Medical Products’ vertebroplasty and bone biopsy lines […]
Benvenue Medical Completes Divestiture of its Vertebral Augmentation Systems Portfolio
SANTA CLARA, Calif.–(BUSINESS WIRE)–Benvenue Medical, Inc., a developer of minimally invasive expandable implant solutions for lumbar fusion, today announced it has completed the divestiture of its vertebral augmentation systems portfolio for an undisclosed amount to IZI Medical Products, LLC. The completion of the sale of this business allows Benvenue Medical to devote its full resources […]
Johnson & Johnson Medical GmbH Acquires Emerging Implant Technologies GmbH to Enhance Global Offering of Interbody Spine Implants
NORDERSTEDT, Germany, Sept. 12, 2018 /PRNewswire/ — Johnson & Johnson Medical Devices Companies*, through its subsidiary Johnson & Johnson Medical GmbH, announced today the acquisition of Emerging Implant Technologies GmbH (EIT), a privately held manufacturer of 3D-printed titanium interbody implants for spinal fusion surgery, based in Wurmlingen, Germany. The products in this portfolio leverage EIT’s […]
Spine Companies to Know with “S” (Part 1)
Today, we display 10 Spine Companies which “S”. Many weeks ago, we started to display the updated list of all the spinal companies worldwide from A to Z.Until now, we have included more than 240 companies (from A to R ). This week we have included some of the the Companies that start with “S” including […]
Amedica Announces Agreement to Sell Spine Business to CTL Medical
SALT LAKE CITY, Sept. 06, 2018 (GLOBE NEWSWIRE) — Amedica Corporation (NASDAQ: AMDA) today announced that it has entered into an asset purchase agreement with CTL Medical, a Dallas, TX-based privately held medical device manufacturer that focuses on the spine implant and instrument market, whereby CTL Medical will acquire all of Amedica’s commercial spine business […]
Is the Spine market Consolidating? 10 acquisitions in 12 months!
September 2, 2018–The Consolidation is the phase in an industry where competitors start to merge. The main objectives for the companies is often to gain a larger portion of overall market share and to take advantage of synergies as Stryker has just done acquiring K2M Inc.Consolidations and mergers are a form of inorganic growth when […]
K2M Group Holdings, Inc. Announces Definitive Agreement To Be Acquired by Stryker Corporation
LEESBURG, Va., Aug. 30, 2018 (GLOBE NEWSWIRE) — K2M Group Holdings, Inc. (Nasdaq: KTWO) (the “Company” or “K2M”), a global leader of complex spine and minimally invasive solutions focused on achieving three-dimensional Total Body Balance™, today announced a definitive merger agreement with Stryker Corporation (“Stryker”, NYSE: SYK) pursuant to which Stryker has agreed to acquire […]
SPINE Companies Catalogue From A to Z: 10 Spine Companies to know with “R”
Over the recent years, global spinal fusion market has been witnessing growth, on account of several driving factors including rising healthcare awareness amongst consumers, surging prevalence of spinal deformities due to accidents along with increasing health expenditure in developing countries. Moreover, ongoing demographic shift towards geriatric population with significant population suffering from inveterate spinal ailments, […]
Caldwell Cassady & Curry Helps Acantha Win $8.2 Million Patent Infringement Verdict Against Medical Device Giant DePuy Synthes
GREEN BAY, Wisc., Aug. 23, 2018 /PRNewswire/ — The Dallas-based intellectual property and business litigation law firm Caldwell Cassady & Curry has won an $8.2 million patent infringement verdict for Acantha LLC against medical device manufacturer DePuy Synthes, a subsidiary of health care giant Johnson & Johnson (NYSE : JNJ ). A jury of five […]
China: Innovative Hybrid PEEK-titanium Expandable Cage for DLIF and OLIF Procedures Demonstrated
THORNTON CLEVELEYS, ENGLAND (PRWEB) AUGUST 14, 2018//The growing interest in PEEK-OPTIMA™ spinal implants in China received further impetus at the 11th Congress of the Chinese Association of Orthopaedic Surgeons (CAOS) held in partnership with the North American Spine Society (NASS). At this event, Fule Science & Technology Development, Beijing, and Invibio Biomaterial Solutions partnered to […]
10 MORE Corpectomy Devices to Know!
Today we have included in our Corpectomy Section (CP) 10 more devices to know. We have included expandable devices as well as other differenciated corpectomy cages (Take into account that some of them may be already out of the market). As we said, there are more than 150,000 spinal fractures in North America* with an […]
NuVasive and Siemens Healthineers partner to transform spine surgery
San Diego and Erlangen, Germany, Aug. 9, 2018 / — NuVasive, Inc. (NASDAQ: NUVA) and Siemens Healthineers today announced a strategic partnership focused on technology development, marketing and commercial activities to advance clinical outcomes in minimally invasive spine surgery. NuVasive is an innovation leader in spine health technology, focused on transforming spine surgery with minimally disruptive, predictable and clinically reproducible procedurally-integrated solutions, […]
Augmedics Begins First-in-Human Clinical Trial of xvision-spine (XVS) Augmented-Reality Surgical Navigation System
YOKNEAM, Israel–(BUSINESS WIRE)–Augmedics has begun a first-in-human clinical trial of its xvision-spine (XVS) augmented-reality surgical navigation system at Sheba Tel Hashomer Medical Center and Asaf Harofeh Medical Center, in Israel. Led by Co-Principal Investigators Dr. Ran Harel and Prof. Yigal Mirovsky, the open label, prospective, single arm, multi-center study will evaluate the safety, performance, accuracy and […]
Centinel Spine Announces that UnitedHealthcare Will Now Cover prodisc L Anterior Lumbar Total Disc Replacement
New York, NY, – Centinel Spine, LLC is pleased to announce that UnitedHealthcare®, the nation’s second largest commercial insurer, has issued a positive medical policy for Lumbar Total Disc Replacement (TDR). This decision affects over 16 million patients covered through UnitedHealthcare, and means that three out of the four largest US commercial providers now have positive […]
SI-BONE, Inc. Announces 23 Commercial Health Insurance Plans Now “Exclusively” Cover Triangular iFuse Implant System® Based on Published Clinical Evidence
SAN JOSE, Calif., Aug. 7, 2018 /PRNewswire/ — SI-BONE, Inc., a medical device company that pioneered the minimally invasive surgical (MIS) treatment of the sacroiliac (SI) joint with the iFuse Implant System® (“iFuse”) announced that 23 commercial health plans have published exclusive positive coverage policies for the triangular iFuse Implant System. These exclusive positive coverage policies provide access to […]
Orthofix Reports Second Quarter 2018 Financial Results
LEWISVILLE, Texas–(BUSINESS WIRE)–Orthofix Medical Inc. (previously Orthofix International N.V.) (NASDAQ:OFIX) today reported its financial results for the second quarter ended June 30, 2018. Net sales were $111.5 million, diluted earnings per share from continuing operations was $0.05 and adjusted earnings per share from continuing operations was $0.42. “During the second quarter, we made excellent progress […]
10 Expandable Corpectomy Devices to Know!
Each year, there are more than 150,000 spinal fractures in North America*. There is an estimated 10,000 to 12,000 spinal cord injuries every year and approximately 39% of these injuries are cause by motor vehicle accidents. These non-elective or trauma surgeries sometimes require additional spinal structural support which is when the Expandable Corpectomy Device are […]
VLIFT Vertebral Body Replacement System
The VLIFT Vertebral Body Replacement System consists of a distractible in situ implant, which enables the surgeon to customize the height of the implant after implantation. Modular end caps and cage extensions, if needed, snap into each end of the implant for quick assembly. The end caps are available in 0° or with angulation to match […]