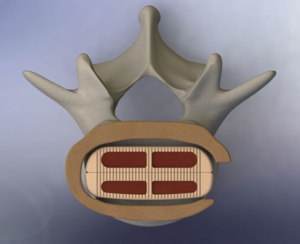
DUBLIN – May 9, 2019 – Medtronic plc (NYSE:MDT), a global leader in medical technology, today announced that it has entered into a definitive agreement pursuant to which it will acquire Titan Spine, a privately-held titanium spine interbody implant and surface technology company. The boards of directors of both companies have unanimously approved the transaction. […]
NEWS
Boston Scientific Announces Agreement To Acquire Vertiflex, Inc.
MARLBOROUGH, Mass., May 9, 2019 /PRNewswire/ — Boston Scientific (NYSE: BSX) today announced that it has entered into a definitive agreement to acquire Vertiflex, Inc., a privately-held company which has developed and commercialized the Superion® Indirect Decompression System, a minimally-invasive device used to improve physical function and reduce pain in patients with lumbar spinal stenosis […]
7 Signs That You Need To Fire Your Outside Product Development Firm
By Lawrence Binder–Chairman at Binder Biomedical, Inc. So, you finally got your medical device project started but you just can’t seem to cross the finish line. From failing prototypes, failing mechanical tests, going over budget, to slipping timelines… your medical device development firm is failing you. If you are an OEM, you start to wonder […]
U.S.Market: Spine Companies to Follow: Part I
According to iData Research, the global spine market is valued at over $14.4 billion USD and is projected to approach $18 billion by 2023. The largest among the regions is undoubtedly the U.S. market, valued at $7.7 billion in 2017. The US TOP PLAYERS The 10 top spine players in the US market are the […]
NuVasive Straightens Up to Start 2019
Dan Caplinger, The Motley Fool May 2, 2019–NuVasive (NASDAQ: NUVA) has been at the forefront of medical advancements in surgical procedures. With its focus on surgery to treat spinal conditions, NuVasive’s minimally disruptive surgical platform and accessories have played a key role in driving more effective treatment options for patients suffering from these health conditions. […]
Top 5 Mistakes Medical Device Inventors Make When They Have A New Idea
By Lawrence Binder/Chairman at Binder Biomedical, Inc. (linkedin.com)–The hardest part of developing a new idea is getting started. But getting started without considering this list could cause major project setbacks. Contact me to discuss how I can help navigate the complicated product development cycle and bring your new idea to life. List Below: Hiring an […]
We are proud and happy to announce that Z-Medical is Sponsor of SPINEMarketGroup in 2019!
Thank you Z-Medical! On behalf of TheSPINEMarketGroup team, we would like to thank you for supporting our site again in 2019 through your PLATINUM sponsorship. About Z-Medical GmbH + Co. KG Based and founded in Tuttlingen in 2010, Z-Medical GmbH + Co. KG is a privately financed and held medical device company that designs, develops, manufactures […]
Stryker announces new Vice President, Chief Legal Officer
Kalamazoo, Michigan, March 27, 2019 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) announced today that Rob Fletcher will be joining the company as Vice President, Chief Legal Officer effective April 22, 2019. Mike Hutchinson will be transitioning from his current role to serve as Advisor to the Chief Executive Officer through May 2020. Mr. Hutchinson has most recently served […]
FDA Approves First Medical Implant with New Superalloy
ATLANTA, March 27, 2019 /PRNewswire/ — MiRus has received FDA 510(k) approval for the MoRe® based Europa™ Pedicle Screw System making it the first FDA approved medical device with this new class of implant material. The Europa™ System received the 2018 Spine Technology Award at the NASS meeting for excellence and innovation in spine surgery. […]
Clinical Results Show Over 50% Reduction of Neurologic Injury from Lateral Spine Surgery with Modular Implant System from VTI
MINNEAPOLIS (PRWEB) MARCH 26, 2019–Vertebral Technologies, Inc. (VTI) announces the presentation of clinical results on their InterFuse® lateral system at the 2019 International Society for the Advancement of Spine Surgery (ISASS) conference in Anaheim, California. VTI is a spine technology company focused on creating modular implants for painful spine conditions that improve minimally invasive surgery (MIS) outcomes. […]
Zimmer Biomet Receives FDA Clearance of ROSA® ONE Spine System for Robotically-Assisted Surgeries
WARSAW, Ind., March 25, 2019 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, today announced U.S. Food and Drug Administration 510(k) clearance of the ROSA® ONE Spine System for robotically assisted minimally invasive and complex spine surgeries, strengthening the Company’s comprehensive ROSA® ONE Brain and ROSA® Knee […]
Fuse Medical Reports Full Year 2018 Financial Results with Record Earnings
RICHARDSON, Texas–(BUSINESS WIRE)–Fuse Medical, Inc., (OTCPINK: FZMD), an emerging manufacturer and distributor of medical devices for the orthopedic and spine marketplace, announced record earnings for the fiscal year ended December 31, 2018. Fuse filed its annual report on Form 10-K with the Securities and Exchange Commission on Thursday, March 21, 2019. 2018 Financial Highlights Net […]
SpineEX Receives Additional FDA Clearance for Innovative Sagittae Lateral Lumbar Interbody Fusion System
FREMONT, Calif., March 25, 2019 (GLOBE NEWSWIRE) — SpineEX, Inc., a medical device company focused on the design, development and marketing of products for spine disorders, received an additional U.S. Food and Drug Administration (FDA) clearance for its innovative Sagittae Lateral Lumbar Interbody (LLIF) Fusion System. This additional 510(k) clearance will enhance SpineEX’s ability to […]
David Paul´s Vision: Robotics in Spine!
(www.forbes.com)–When David C. Paul traveled to Phoenix in 2013, he saw the future of spinal surgery: a robot prototype called the Excelsius GPS. Nicholas Theodore, one of the robot’s inventors, remembers Paul being immediately impressed. “This is going to change everything,” Paul said, according to Theodore. A few months later, Paul bought Theodore’s company, Excelsius Surgical—and the […]
Medicrea Reports 2018 Annual Results
LYON, France & NEW YORK–(BUSINESS WIRE)–The Medicrea Group (Euronext Growth Paris: FR0004178572-ALMED ; OTCQX Best Market –MRNTF), pioneering the transformation of spinal surgery through artificial Intelligence, predictive modeling and patient specific implants with its UNiD™ ASI (Adaptive Spine Intelligence) proprietary software platform, services and technologies, publishes its 2018 IFRS annual results, as audited and approved […]
Astura Medical Reports 104% Annual Revenue Growth in 2018
CARLSBAD, CA – March 22, 2019 – Astura Medical, a high-growth, innovative spine technology company, today reported an annual revenue increase of 104% for the full year ending Dec. 31, 2018. “We’re proud of the successful performance by our team in 2018. To more than double our revenue for the third consecutive year further solidifies […]
Ozop Surgical Corp Provides Corporate Update and Outlook for 2019
WEST PALM BEACH, FL / ACCESSWIRE / March 18, 2019 / Ozop Surgical Corp. (OTCQB: OZSC), a provider of premium surgical devices in the rapidly growing field of minimally invasive spine surgery, released the following corporate update and outlook for 2019 from Michael Chermak, Chief Executive Officer and Chairman. As a way of introduction; Ozop Surgical […]
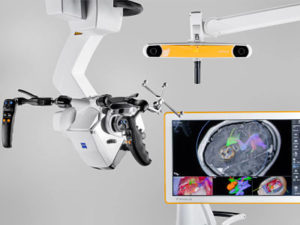
Brainlab Acquires Robotics Platform Company Medineering
MUNICH–(BUSINESS WIRE)–Brainlab, a global pioneer of software-driven medical technology, announced today the acquisition of Medineering, a developer of application-specific robotic technologies. With this move, Brainlab is driving the democratization of digital surgery with scalable solutions that expand clinical frontiers. This strategic move increases the depth of the Brainlab portfolio in cranial surgery and strengthens its […]
RTI Surgical Enrolls First Patient in FORTE Clinical Study of Fortilink® Interbody Fusion Device with TETRAfuse® 3D Technology
DEERFIELD, Ill., March 20, 2019 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (NASDAQ: RTIX), a global surgical implant company, today announced it enrolled the first patient in its Clinical Evaluation of Fortilink® Interbody Fusion Device with TETRAfuse® 3D Technology in Subjects with Degenerative Disc Disease (FORTE) study. FORTE is a prospective, multicenter post-market evaluation of […]
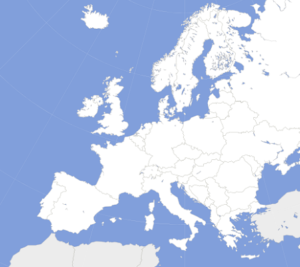
Europe Spinal Implants Market is Anticipated to Reach US$ 4 Billion by 2024.Which are the main Players?
According to Market Research, the Europe Spinal Implants Market is expected to exceed more than US$ 3.5 Billion by 2024 at a CAGR of 5.5%. The major driving factors of Europe Spinal Implants Market are as follows: Rise in obese population Rise in Government healthcare expenditure Cost effective spinal devices Rising demand in minimally invasive spine surgery […]
Centinel Spine Appoints Dirk M. Kuyper and Gregory Rainey to its Board of Directors
NEW YORK, March 19, 2019 /PRNewswire/ — Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced the appointment of Dirk M. Kuyper and Gregory Rainey as members of the Company’s Board of Directors. “I am thrilled to welcome both Dirk and Gregory to the Centinel board,” says Centinel Spine Chairman & CEO, John Viscogliosi. “Both individuals bring […]
Implanet: FDA Clearance for JAZZ Cap® System
BORDEAUX, France & BOSTON: IMPLANET (Paris:ALIMP) (OTCQX:IMPZY) (Euronext Growth: ALIMP, FR0010458729, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee surgery implants, has announced that it has received 510(k) authorization from the Food and Drug Administration (FDA) for its JAZZ Cap® System, designed to meet the constraints of vertebral […]
75 acquisitions in the last 3 years: Are Acquisitions the only way to Grow?
Are Acquisitions the only way to Grow? NO!… but are probably the quickest way! What about Organic Grow? Organic growth is slow and most of the companies are very impatient when it comes to seeing results. Today, is hard to grow in the spinal market. It may take a lot of work and time.The approaches […]
Stryker acquires OrthoSpace, Ltd.
Kalamazoo, Michigan, March 14, 2019 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) announced today it has completed the acquisition of OrthoSpace, Ltd., a privately held company founded in 2009 and headquartered in Caesarea, Israel, in an all cash transaction for an upfront payment of $110 million and future milestone payments of up to an additional $110 million. […]
SpineGuard Reports Solid Improvements in Its Full-Year 2018 Financial Results
PARIS & SAN FRANCISCO–SpineGuard (FR0011464452 – ALSGD)(Paris:ALSGD), an innovative company that deploys its DSG® real time digital technology for surgical guidance intended to secure and streamline skeletal implant placement, reported today its full-year 2018 financial results as approved by the Board of Directors on March 13, 2019. € thousands – IFRS audited Dec 31, 2018 […]
AxioMed Announces CE Mark for Cervical Freedom® Total Disc Replacement
BOSTON (PRWEB) MARCH 14, 2019 AxioMed announced today the issuance of the CE Mark approval for their viscoelastic disc replacement. The CE Mark approval was received as a result of an accredited Notified Body assessment of the company’s complete portfolio of biocompatibility and biomechanical cervical disc testing and supporting clinical data regarding the viscoelastic AxioMed technology. AxioMed […]
Medacta Showcases Surgical Innovation With MIKA, MectaLIF Anterior, and MySpine MC on Display at AAOS 2019
LAS VEGAS–(BUSINESS WIRE)–Celebrating its 20th anniversary in 2019, Medacta International is showcasing its robust personalized medicine portfolio this week at the American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting. The recently unveiled Medacta Individualized Kinematic Alignment (MIKA) instrumentation and associated surgical technique, MectaLIF® Anterior Interbody Fusion Device, and MySpine® MC Surgical Guides, among other key […]