DALLAS, Feb. 12, 2020 /PRNewswire/ — 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design that utilizes its proprietary Truss Implant Technology™, announced today that the company achieved record revenues in 2019. Additionally, in Q4 2019, 4WEB recorded the company’s largest quarterly revenue to date. “With investments in product development, clinical research, […]
NEWS
CTL Amedica Corp Granted Patent for Screw Offset Blocking Mechanism: Newly Patented Tech Implemented in MONET Anterior Cervical Fusion System
DALLAS (PRWEB) FEBRUARY 11, 2020–CTL Amedica Corporation has been granted an official patent from the United States Patent and Trademark Office for a screw offset blocking mechanism, which has been thoughtfully designed for and implemented in the company’s newly released MONET™ Anterior Cervical Fusion System. CTL Amedica plans to implement the robust blocking mechanism in future […]
5-Year Follow-Up Outpatient of L4-L5 Fixation with Inspan Device for Degenerative Spinal Stenosis Demonstrates Improved Results
BOSTON (PRWEB) FEBRUARY 11, 2020–INSPAN LLC (a privately-owned company) is pleased to announce the successful results from a long-term follow-up clinical study of outpatient L4-L5 lumbar interspinous fixation for degenerative spinal stenosis using the Inspan interspinous fixation device (INSPAN LLC). Unlike extension block design, the Inspan device fixates the spine to allow for immediate stability, distraction, […]
10 Cervical Cages to Know…!
According to reportsweb.com The global Cervical Interbody Fusion Cages market is valued at 820 million US$ in 2017 and will reach 1120 million US$ by the end of 2025, growing at a CAGR of 4.0% during 2018-2025. In the market there are more than 100 cervical cages including PEEK, 3D Titanium, or stand-alone. Most of […]
SeaSpine® and 7D Surgical Announce Entry into a Strategic Alliance Agreement to Distribute 7D Surgical’s Flagship Machine-Vision Image Guided Surgery (MvIGS) Platform
CARLSBAD, Calif. and TORONTO, Feb. 07, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, and 7D Surgical, Inc., a privately-held Toronto-based company developing advanced image guidance technologies and machine-vision-based registration algorithms to improve surgical workflow and patient care, today announced that they have entered into a strategic […]
New Spine Catalogue 2020: Coming Soon!
We are preparing the New Spine Catalogue 2020! We hope to have the final version by next week because we still have to incorporate more companies and to revise all the included ones. In the Spine Catalogue 2019, we accounted for 410 companies but we still missed many… This time we would like to have […]
Globus Medical Announces First Case with Next Generation 3D Printed Spine Implant
AUDUBON, Pa., Feb. 04, 2020 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company announced Dr. Joshua S. Rovner became the first spine surgeon to implant HEDRON IA, a 3D printed integrated ALIF spacer that leverages anchors or screws for vertebral body fixation. The minimally invasive outpatient procedure was recently performed at […]
Osseus Fusion Systems Has Officially Announced the Alpha Launch Of Aries™-TS
DALLAS, TX – On February 4th, 2020, Osseus Fusion Systems announced the official Alpha Launch of Aries™-TS, its 3D printed transforaminallumbar interbody fusion device. “In January, we celebrated the one-year anniversary of the firstimplantation of the Aries™-L lateral lumbar interbody fusion device so we are very excited to follow it up with the launch of the Aries™-TS. As a company committed to helping those in need of relief from chronic lumbar […]
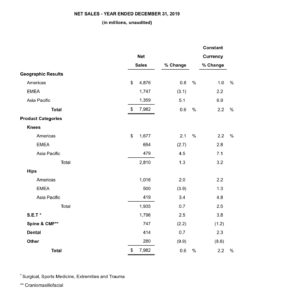
Zimmer Biomet Announces Fourth Quarter and Full-Year 2019 Financial Results
WARSAW, Ind., Feb. 4, 2020 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH) today reported financial results for the quarter and year ended December 31, 2019. The Company reported fourth quarter net sales of $2.126 billion, an increase of 2.6% over the prior year period, and an increase of 3.2% on a constant currency basis. Net sales for […]
Nikolaus Beyer Appointed as Safe Orthopaedics’ Chief Commercial Officer
Safe Orthopaedics (Paris:ALSAF) (FR0013467123 – ALSAF), a company marketing innovative ready-to-use technologies (single-use implants and instruments) for spinal diseases, delivering the safest treatment of spinal fractures, announces the appointment of Nikolaus Beyer as Chief Commercial Officer. Nikolaus Beyer has 25 years of experience in the market of spinal surgery, within groups such as J&J, Stryker […]
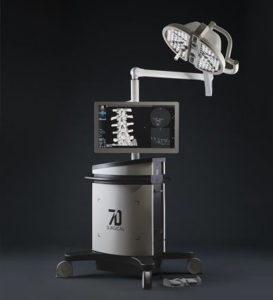
7D Surgical Expands Spine and Cranial Technology Offering
TORONTO, Feb. 3, 2020 /PRNewswire/ — 7D Surgical announced today important additions to its cranial and spinal product lines. The company has received both 510(k) clearance from the U.S. Food and Drug Administration (FDA) and a medical device license from Health Canada for its Cranial Biopsy Kit, which allows neurosurgeons to use image guidance to accurately target brain […]
Aurora Spine Announces Closing of First Tranche of Private Placement Financing
CARLSBAD, Calif., Feb. 03, 2020 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSX VENTURE:ASG) is pleased to announce that it has closed the first tranche of up to approximately CDN$2.2 million non-brokered private placement offering (the “Offering”). Under the first tranche of the Offering, the Company has issued 5,500,000 units (the […]
10 Porous TLIF cages to Know…!
According to Allied Market Research, the global interbody fusion cage market was valued at $1,818 million in 2016, and is expected to reach $2,309 million by 2023 at a CAGR of 3.4% during the forecast period. Rise in geriatric population and increase in incidences of spinal injuries and sports injuries drive the growth of the […]
“Alphatec’s New Product Introductions are Minor Improvements” We do not Agree…
A few days ago, I found an article in seekingalpha.com related to the evolution and future of Alphatec Spine. In its analysis aimed at potential investors in this Company, different influential factors in its progress were discussed. Among them, they commented on the lack of technological innovation and questioned their products differentiation by writting that […]
DeGen Medical Expands Operations to Charleston, South Carolina
Florence, SC, Jan. 30, 2020 (GLOBE NEWSWIRE) — DeGen Medical, Inc., an innovator in the design and manufacturing of spinal implant technology, is pleased to announce its expansion to Charleston, South Carolina. The new facility is in the heart of the downtown commercial business district at the corner of King Street and Market Street. This new […]
Centinel Spine and Tiger Woods Create Behind-the-Scenes Look at Surgery and Recovery
NEW YORK, Jan. 30, 2020 /PRNewswire/ — Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced the planned February filming of a three to four minute video detailing the story behind 15-Time PGA Tour Majors Winner Tiger Woods’ remarkable recovery from incapacitating back pain—and his successful spinal fusion surgery through the support […]
Atlas Spine Launches its V3 Guided Segmental Cervical Plating System, Expands Solutions for Cervical Deformity
Palm Beach County, FL, January 28, 2020 — Atlas Spine Inc., a spinal implant company based in Jupiter, Florida, announced today the launch of its V3 Guided Segmental Plating System, expanding the company’s disruptive technology solutions for treating complex deformity and degenerative conditions of the cervical spine. Cervical spine pathologies afflict hundreds of thousands of people each year. The general growth of an aging population […]
Zavation Facilitates 10,000+ Kyphoplasty Procedures in 2019 With Novel Curved Systems
FLOWOOD, Miss., Jan. 28, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, acquired PanMed US Corp. (“PanMed”) in September 2018. This acquisition added a full suite of minimally invasive products, including PanMed’s curved platform consisting of the novel InterV Flex and CurvePlus kyphoplasty […]
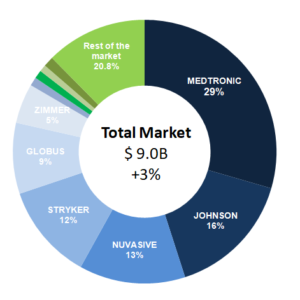
Today´s Spine Market Shares Estimate and Full year Revenues
In 2019, the total spine market was valued at $9 billion worldwide, growing 3% related to 2018. The growing demand for MIS spine surgeries, the introduction of 3D printed implants and the availability of technology such as robotics have been some of the emerging trends, which have contributed to the growth of the global spinal […]
Robert Pace Tabbed As New Osseus CEO
January 27, 2020 – Dallas, Texas – Osseus Fusion Systems, LLC—Osseus Fusion Systems is pleased to announce today that Rob Pace has taken over as its new Chief Executive Officer. As a strong team builder with expertise in improving organizational efficiency, strategic planning, brand cultivation, sales operations, and business execution, Rob’s leadership has allowed his […]
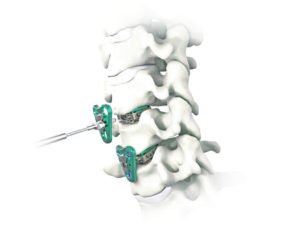
SpineCraft announces the launch of the ASTRA Occipito-Cervico-Thoracic (OCT) Spine System
January 24, 2020 –The ASTRA-OCT System is a comprehensive set of implants and instruments to stabilize the spine in patients undergoing posterior cervical fusion. The system was developed to address unmet needs in complex posterior spine fixation procedures, revisions and extensions. ASTRA-OCT includes a wide range of screw, connector and rod options for both the […]
First Surgeries with SteriSpine™ CC & LC Completed in Japan
Éragny-sur-Oise, France, January 23, 2020 – SAFE ORTHOPAEDICS (FR0013467123 – ALSAF), a company marketing innovative ready-to-use technologies (single-use implants and instruments) for spinal diseases, delivering the safest treatment of spinal fractures, announces that ten surgeries have been completed in Japan since SteriSpine CC (Cervical cages) & LC (Lumbar cages) have been approved in October, 2019. […]
Centinel Spine Announces Continued Partnership with Performance Tech Motorsports
NEW YORK, Jan. 23, 2020 /PRNewswire/ — Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced that its current partnership with Performance Tech Motorsports as Title Sponsor for the team will continue through the 2020 IMSA WeatherTech SportsCar Championship season after successful programs the past several years. Since 2018, […]
Johnson & Johnson Reports 2019 Fourth-Quarter And Full Year Results
NEW BRUNSWICK, N.J., Jan. 22, 2020 /PRNewswire/ — Johnson & Johnson (NYSE: JNJ) today announced results for fourth-quarter and full year 2019. “We delivered strong underlying sales and earnings growth in 2019, driven by the strength of our Pharmaceutical business, accelerating performance in our Medical Devices business and improved profitability in our Consumer business,” said […]
EOS imaging Announces First Installation of EOSedge™ in North America
PARIS–(BUSINESS WIRE)–Regulatory News: EOS imaging (Paris:EOSI) (Euronext, FR0011191766 – EOSI – Eligible PEA – PME), a leader in 2D/3D orthopedic medical imaging and software solutions for 3D anatomical modeling and surgical planning, today announced the first installation of its new EOSedge™ system in North America at CHU Sainte-Justine Mother and Child University Hospital Centre in […]
Inspired Spine Announces The Completion Of 1,000 OLLIF Procedures
BURNSVILLE, Minn., Jan. 20, 2020 /PRNewswire/ — Inspired Spine reached the milestone of completing 1,000 Oblique Lateral Lumbar Interbody Fusion (OLLIF) procedures. This minimally invasive surgical technique is designed to improve the performance of lumbar interbody fusions by delivering superior outcomes including less patient recovery time and minimal blood loss, while requiring a procedure duration as short as […]
Why RTI Surgical has sold their OEM business?
RTI’s board has unanimously approved the deal to sell its OEM business to a leading European private equity firm for a total of $490 million, with $480 million of the transaction paid in cash.Proceeds from the deal will be used to pay down debt and recapitalize the remaining business. CEO Camille Farhat said: “The sale of the […]