Amplify Surgical, Inc., today announces that the company reached the milestone of treating 300 spinal levels with the dualX Expanding Interbody Fusion System. The landmark case was performed by Dr. Ali Najafi at Fresno Heart and Surgical Hospital in Fresno, CA. After the case Dr. Najafi noted, “dualX has completely changed the game for expandable […]
NEWS
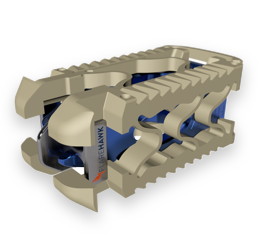
Clinical Study Demonstrates Favorable Patient Outcomes with the FlareHawk® Expandable Cage
PALM BEACH GARDENS, Fla., March 03, 2020 (GLOBE NEWSWIRE) — Integrity Implants Inc., a privately held medical device company dedicated to delivering innovative solutions for spine surgery, today announced positive data from a retrospective study demonstrating favorable fusion efficacy with its FlareHawk® interbody implant. The study, “Transforaminal/Posterior Lumbar Interbody Fusion with the FlareHawk Expandable Interbody Fusion Device,” […]
Implanet Steps Up Development in Germany and Consolidates Its Organization
IMPLANET (Paris:ALIMP) (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, is reinforcing its business operations in Germany, a strategic market for the company. Implanet, which operates in Germany via its subsidiary based in Frankfurt (Implanet GmbH), has appointed Stephan Collardey as Country Manager […]
AO, icotec Join Forces on New Spinal Stabilization System
DAVOS, Switzerland, March 3, 2020 /PRNewswire/ — The AO Foundation and icotec ag announce the successful agreement and kick-off for the joint development of a new spinal stabilization system based on icotec’s proprietary BlackArmor® Carbon/PEEK composite implant material. “The AO is delighted to partner with icotec ag, and to leverage its game changing technology platform based on […]
NuVasive to Participate at Spine Summit 2020
SAN DIEGO, March 2, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced it will participate at Spine Summit 2020, the 36th Annual Meeting of the Section on Disorders of the Spine and Peripheral Nerves on March 5-8, 2020 at The Cosmopolitan of Las Vegas. […]
OrthoPediatrics Corp. to Highlight its Pediatric Scoliosis Solutions at 6th Annual International Children’s Spine Symposium (ICSS)
WARSAW, Ind., March 02, 2020 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics”) (NASDAQ: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, today announced it will attend the 6th Annual International Children’s Spine Symposium (ICSS), which will take place March 13-14, 2020 in Orlando, Florida. The meeting offers a comprehensive program of lectures given an outstanding […]
SINTX Technologies Announces Appointment of Mark I. Froimson, M.D., to Its Board of Directors
SALT LAKE CITY, UT, March 02, 2020 (GLOBE NEWSWIRE) — SINTX Technologies, Inc. (NASDAQ: SINT) (“SINTX” or the “Company”), an original equipment manufacturer (OEM) ceramics company focused on silicon nitride applications, today announced the appointment of Mark I. Froimson, M.D. to its board of directors. Dr. Froimson is currently a Principal at Riverside Health Advisors, […]
10 OCT Spinal Systems to Know…!
The posterior cervical fusion is a type of cervical fusion surgery, that accounts the 15% to the cervical devices market share. Although it is still an small segment of the overall Spinal market, it is growing due to the increasing prevalence of osteoarthritis and rheumatoid and the rising aging population. The estimated market value of […]
ATEC Announces Agreement to Acquire EOS imaging
CARLSBAD, Calif., Feb. 28, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today that it has entered into an agreement to acquire EOS imaging, SA, for a purchase price of up to $88 million, plus debt retirement […]
Spine Catalogue 2020: 400 Companies Listed!
The Spine market accounts in 2020 for $9,5 billion worldwide, with an estimated increase of 2,5% vs. 2019. Analysts are forecasting a range of 1% to 2,5% growth for this market in the next few years because of price pressures and stricter insurance companies policies. The main market players are:Medtronic, J&J, Stryker, Nuvasive, Globus Medical […]
DeGen Medical Announces First Minimally Invasive Case with Dr. Peter Derman at Texas Back Institute
Florence, SC, Feb. 26, 2020 (GLOBE NEWSWIRE) — DeGen Medical, Inc. an innovator in the design and manufacturing of spinal implant technology announced that Dr. Peter Derman became the first surgeon to implant the E3 MIS pedicle screw system at Texas Back Institute (TBI). The minimally invasive procedure was recently performed at Texas Health Center for Diagnostics and […]
SeaSpine Announces Fourth Quarter and Full-Year 2019 Results and Reaffirms 2020 Revenue Guidance
CARLSBAD, Calif., Feb. 26, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today financial results for the three-months and full-year ended December 31, 2019 and reaffirmed revenue guidance for 2020. Summary Fourth Quarter 2019 Financial Results and Recent Accomplishments Revenue of $43.7 million, an increase […]
RTI Surgical® Releases Data on the SImmetry® System Showing 98% of Treated SI Joints Demonstrate Evidence of Fusion
DEERFIELD, Ill., Feb. 26, 2020 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (Nasdaq: RTIX), a global surgical implant company, will showcase its expanding body of clinical data on the SImmetry® Sacroiliac Joint Fusion System at the 2020 International Society for the Advancement of Spine Surgery (ISASS) meeting in San Juan, Puerto Rico, from February 26-28. The […]
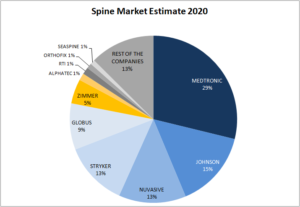
Which is the Spine Market Estimate for 2020?
The Spine market accounts in 2020 for $9,5 billion worldwide, with an estimated increase of 2,5% vs. 2019. Analysts are forecasting a range of 1% to 2,5% growth for this market in the next few years because of price pressures and stricter insurance companies policies. Our estimate of the 10 top spine players’ market share […]
NuVasive Announces Fourth Quarter and Full Year 2019 Financial Results
SAN DIEGO – February 20, 2020 – NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced financial results for the quarter and full year ended Dec. 31, 2019. Fourth Quarter 2019 Revenue increased 7.6% to $310.4 million, or 7.8% on a constant currency basis; […]
Simplify Medical Selected to Present Two Podium Presentations at the 20th ISASS Annual Conference
SUNNYVALE, Calif., Feb. 20, 2020 (GLOBE NEWSWIRE) — Simplify Medical Pty Ltd, maker of the Simplify® cervical artificial disc, announced today the International Society for the Advancement of Spine Surgery (ISASS) has selected two abstracts on the Simplify Disc for podium presentations at the 20th Annual Conference. The largest international society meeting focused exclusively on spine surgery is […]
Globus Medical Reports Fourth Quarter and Full Year 2019 Results
AUDUBON, Pa., Feb. 20, 2020 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, today announced its financial results for the fourth quarter and year ended December 31, 2019. Fourth Quarter: Worldwide sales increased 8.0% as reported to $211.7 million Fourth quarter net income was $45.5 million, or 21.5% of sales Diluted EPS […]
Genesys Spine is pleased to announce the launch of a Sacroiliac Joint Fusion System.
Thursday, February 20, 2020–Austin, TX: The Genesys Spine Sacroiliac Joint Fusion System consists of partially threaded and fully threaded implants designed to secure the sacroiliac joint and minimize micro-motion enabling bony fusion. Advantages of the Genesys Spine Sacroiliac Joint Fusion System include: Dual thread designs incorporate a differential pitch for controlled compression across the joint. […]
Biomechanical Analysis of Inspan Spinous Process Fixation Alone in The Lumbar Spine Demonstrates Positive Results
INSPAN LLC (a privately-owned company) is pleased to announce the positive results from a biomechanical analysis of spinous process fixation alone or with facet screws in the lumbar spine using the Inspan interspinous fixation device (INSPAN LLC). Unlike extension block design, the Inspan device fixates the spine to allow for immediate stability, distraction, decompression, and […]
Medtronic Reports Third Quarter Financial Results
DUBLIN, Feb. 18, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced financial results for its third quarter of fiscal year 2020, which ended January 24, 2020. The company reported third quarter worldwide revenue of $7.717 billion, an increase of 2.3 percent as reported and 2.6 percent on an organic basis, which adjusts for a $46 million negative impact […]
Oxford Performance Materials’ OsteoFab® 3D Printed PEKK Technology Focus of Study Published in The Spine Journal
SOUTH WINDSOR, CONN. (PRWEB) FEBRUARY 17, 2020–Oxford Performance Materials, Inc. (OPM), an industry leader in advanced materials science and high-performance additive manufacturing (HPAM®), announced today the publication of “A Comparative Study of Three Biomaterials in an Ovine Defect Model: A TETRAfuse® PEKK Study” in The Spine Journal.1,2,3 This study examined the in vivo material characteristics of […]
Spineart announces acquisition of Eden Spine Europe’s assets
Spineart SA is pleased to announce that it has recently acquired assets from Eden Spine Europe SA, a Geneva based company specializing in spinal implants. This acquisition complements Spineart’s existing portfolio in particular with a cervico-thoracolumbar VBR system and an Antero-Lateral Plate. About SpineArt Spineart is a privately held medical device company focused on simplifying the […]
6 Expandable Corpectomy Devices to Know…!
Spine fractures sometimes may require additional spinal structural support with an implant such as a Mesh, a peek cage or an expandable corpectomy device. Another indication or use for these Devices is in the treatment of cancer patients. If the cancer metastasizes or spreads in the spine, the surgeon may opt to remove the affected […]
Medtronic to Advance Solutions and Capabilities in Surgical Data and Analytics with Acquisition of Digital Surgery
DUBLIN, Feb. 13, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced the acquisition of Digital Surgery, a privately-held pioneer in surgical artificial intelligence (AI), data and analytics, and digital education and training. The acquisition of Digital Surgery will strengthen the Medtronic robotic assisted surgery platform and has applicability […]
It’s not just about fusion anymore! An Expandable Cervical Interbody Cage to know!
As Matt Baynham, Atlas’ CEO said last year when launching the HiJak cage, “It is not just about fusion anymore“. As studies and clinical evidence continue to show, the value of restoring lordosis and global sagittal balance to the cervical spine is becoming more widely recognized and prioritized by spine surgeons today. The benefits that […]
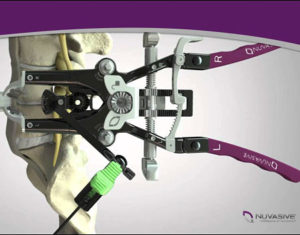
Results Validating the Versatility of NuVasive’s MaXcess® Retractor in Prone Single-Position XLIF® and Posterior Fixation Published in The European Spine Journal
SAN DIEGO, Feb. 13, 2020 /PRNewswire/ –NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the results of the study, “Prone single-position extreme lateral interbody fusion (pro-XLIF): preliminary results,” in The European Spine Journal, validating the versatility of NuVasive’s MaXcess® retractor in prone, single-position eXtreme Lateral […]
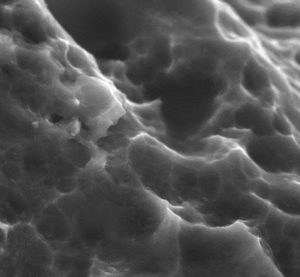
Nanocoated PEEK cages improve osseointegration in PLIF procedure
Investigators have found that nanocoated polyetheretherketone (PEEK) cages for posterior lumbar interbody fusion (PLIF) achieve a better fusion rate than uncoated PEEK cages at one year follow-up—while also having similar safety and efficacy outcomes. These are the conclusions of a randomised controlled trial, conducted by Karel Willems (Department of Orthopedic Surgery, AZ Delta, Roselare, Belgium) […]