LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced the appointment of Patrick Fisher as President of the Orthofix Global Orthopedics business. In this role, Fisher will serve on the company’s Executive Leadership team, reporting to Orthofix President and CEO Massimo Calafiore. Fisher joins … [Read more...] about Patrick Fisher Joins Orthofix as President of Global Orthopedics Business
NEWS
Bioretec Ltd’s half-year report January-June 2024: RemeOs™ trauma screw controlled launch in the U.S. yielded expected positive clinical results
TAMPERE, Finland, Aug. 15, 2024 /PRNewswire/ -- This announcement is a summary of Bioretec Ltd's half-year report January-June 2024. The complete half-year report with tables is attached to this release as a pdf file and available at the company's web pages at https://bioretec.com/investors/investors-in-english/releases. April-June 2024 in brief January-June … [Read more...] about Bioretec Ltd’s half-year report January-June 2024: RemeOs™ trauma screw controlled launch in the U.S. yielded expected positive clinical results
Zavation Medical Products, LLC ranks as one of the fastest-growing private companies in America
FLOWOOD, Miss., Aug. 13, 2024 /PRNewswire/ -- Inc. revealed today that Zavation Medical Products, LLC ranks as one of the fastest-growing private companies in America. The prestigious ranking provides a data-driven look at the most successful companies within the economy's most dynamic segment—its independent, entrepreneurial businesses. The Inc. 5000 … [Read more...] about Zavation Medical Products, LLC ranks as one of the fastest-growing private companies in America
KIC Ventures Strengthens Corporate Compliance by Hiring Leading Healthcare Law Firm
KIC Ventures partnered with Southern Health Lawyers, LLC to enhance corporate compliance beginning in July 2024. This collaboration reinforces KIC Ventures' prioritized commitment to adhering to healthcare regulations. Southern Health Lawyers, a top healthcare law firm since 2012, will provide expert legal guidance. KIC Ventures continues to meet Sunshine Act requirements … [Read more...] about KIC Ventures Strengthens Corporate Compliance by Hiring Leading Healthcare Law Firm
Spectrum Dynamics Medical and DEEPDENSE Medical Enter into a Partnership to Revolutionize AI in Cardiac and Spine Disease Imaging and Treatment
SARASOTA, Fla., Aug. 13, 2024 /PRNewswire/ -- Spectrum Dynamics Medical, a leading innovator in digital SPECT-CT imaging solutions, announced that its partnering with DEEPDENSE Medical, developing innovative AI-based clinical applications to revolutionize Cardiac and Spine disease analysis. This collaboration aims to enhance the visualization and analysis of cardiac and … [Read more...] about Spectrum Dynamics Medical and DEEPDENSE Medical Enter into a Partnership to Revolutionize AI in Cardiac and Spine Disease Imaging and Treatment
Stryker announces definitive agreement to acquire care.ai, a leading virtual care and ambient intelligence solutions platform
Portage, Michigan, Aug. 12, 2024 (GLOBE NEWSWIRE) -- Stryker (NYSE: SYK), a global leader in medical technologies, announced today a definitive agreement to acquire care.ai, a privately held company specializing in delivering AI-assisted virtual care workflows, smart room technology and ambient intelligence solutions. The acquisition will strengthen Stryker’s growing healthcare … [Read more...] about Stryker announces definitive agreement to acquire care.ai, a leading virtual care and ambient intelligence solutions platform
Kuros Biosciences Reports First Half of 2024 Results
Schlieren (Zurich), Switzerland, August 8, 2024 – Kuros Biosciences (“Kuros” or the “Company”) a leader in next generation bone healing technologies, today announced financial results for the first half of 2024. Financial Highlights Regulatory, Clinical & Commercial Highlights Outlook Events after the reporting period The group has no significant … [Read more...] about Kuros Biosciences Reports First Half of 2024 Results
Globus Medical Reports Second Quarter 2024 Results
AUDUBON, Pa., Aug. 06, 2024 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the quarter ended June 30, 2024. “I’m pleased with the quarterly results, demonstrating our team’s strong performance as we continue to execute our long-term growth strategy, deliver on NuVasive … [Read more...] about Globus Medical Reports Second Quarter 2024 Results
Spineology® Announces $25 Million Equity Financing
ST. PAUL, Minn.--(BUSINESS WIRE)--Spineology, the medical device company pioneering Conform and Expand™ spinal fusion technology, today announced that it has raised $25 million in Series AA financing led by SV Health Investors, with participation from 1315 Capital and its existing investor, RC Capital. “We are thrilled to welcome SV Health Investors and 1315 Capital. This … [Read more...] about Spineology® Announces $25 Million Equity Financing
SI-BONE, Inc. Reports Financial Results for the Second Quarter 2024
SANTA CLARA, Calif., Aug. 05, 2024 (GLOBE NEWSWIRE) -- SI-BONE, Inc. (Nasdaq:SIBN), a medical device company dedicated to solving sacropelvic disorders, today reported financial results for the quarter ended June 30, 2024. Second Quarter 2024 Financial Highlights (comparisons are to the prior year period) Recent Operational Highlights (comparisons are to the … [Read more...] about SI-BONE, Inc. Reports Financial Results for the Second Quarter 2024
OrthoPediatrics Corp. Reports Second Quarter 2024 Financial Results and Reaffirms Full Year 2024 Revenue Guidance
WARSAW, Ind., Aug. 05, 2024 (GLOBE NEWSWIRE) -- OrthoPediatrics Corp.(“OrthoPediatrics” or the “Company”) (Nasdaq: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, today announced its financial results for the second quarter ended June 30, 2024. Second Quarter 2024 and Recent Business Highlights David Bailey, … [Read more...] about OrthoPediatrics Corp. Reports Second Quarter 2024 Financial Results and Reaffirms Full Year 2024 Revenue Guidance
Carlsmed Announces Favorable Medicare Reimbursement Decision for Spinal Fusions with Custom-Made Anatomically Designed Devices
CARLSBAD, Calif.--(BUSINESS WIRE)--Carlsmed, the leader in personalized spine surgery, announced today that in the Hospital Inpatient Prospective Payment System Final Rule for fiscal year 2025, the Centers for Medicare & Medicaid Services (CMS) assigned spinal fusion cases with aprevo® custom-made anatomically designed interbody fusion devices to the highest level of the … [Read more...] about Carlsmed Announces Favorable Medicare Reimbursement Decision for Spinal Fusions with Custom-Made Anatomically Designed Devices
NanoHive Medical Announces $7M Series C Financing and Change to Directors Composition
BOSTON, MA, August 5, 2024 - NanoHive Medical, a leader in 3D-printed titanium spinal interbody fusion devices, announces a $7M Series C Financing to capitalize the company’s rapid growth and a priority on company-building to profitability. Outcome Capital served as Strategic and Financial Advisor to NanoHive. The primary use-of-proceeds include: (i) expanding the U.S. … [Read more...] about NanoHive Medical Announces $7M Series C Financing and Change to Directors Composition
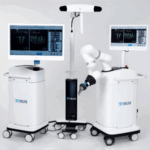
DePuy Synthes Launches its First Active Spine Robotics and Navigation Platform
PALM BEACH GARDENS, Fla., Aug. 2, 2024 /PRNewswire/ -- Today, Johnson & Johnson MedTech* announced that DePuy Synthes, The Orthopaedics Company of Johnson & Johnson**, is launching a proprietary dual-use robotics and standalone navigation platform developed in collaboration with eCential Robotics. The system, called the VELYS™ Active … [Read more...] about DePuy Synthes Launches its First Active Spine Robotics and Navigation Platform
MiRus Receives Breakthrough Device Designation for Spine Implant
ATLANTA, July 31, 2024 /PRNewswire/ -- MiRus has received Breakthrough Device Designation from the FDA for the EUROPA®Posterior Cervical System, based on it's proprietary rhenium alloys, for treatment of the cervical and upper thoracic spine. The EUROPA® PCF system is built around a 2.9 mm MoRe rod which is much smaller than current commercial … [Read more...] about MiRus Receives Breakthrough Device Designation for Spine Implant
Stryker reports second quarter 2024 operating results
Portage, Michigan, July 30, 2024 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) reported operating results for the second quarter of 2024: Second Quarter Results "We delivered another quarter of strong organic sales growth," said Kevin A. Lobo, Chair and CEO. "We remain committed to our margin expansion goals and are excited about our product and M&A … [Read more...] about Stryker reports second quarter 2024 operating results
ATEC Reports Second Quarter 2024 Financial Results And Raises Full-Year Guidance
CARLSBAD, Calif.--(BUSINESS WIRE)-- Alphatec Holdings, Inc. (NASDAQ:ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended June 30, 2024, and recent corporate highlights. Recent Highlights “In the second quarter, ATEC’s procedural thesis perpetuated best-in-class top … [Read more...] about ATEC Reports Second Quarter 2024 Financial Results And Raises Full-Year Guidance
Life Spine Announces FDA 510(k) Clearance for the ARx® SAI Spinal Fixation System
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the ARx SAI (Sacral Alar Iliac) Spinal Fixation System. The ARx SAI Spinal Fixation System … [Read more...] about Life Spine Announces FDA 510(k) Clearance for the ARx® SAI Spinal Fixation System
SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand
ONTARIO, Calif., July 31, 2024 /PRNewswire/ -- Leading augmented reality (AR) medical technology company SURGLASSES today announced that, in collaboration with exclusive distributor Goodlifeintertrade.co. Ltd., it has successfully registered and clinically implemented its AR-based Caduceus S surgical navigation system in Thailand. This groundbreaking system is … [Read more...] about SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand