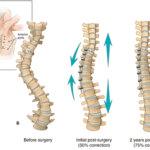
NORWALK, Conn., Sept. 13, 2024 /PRNewswire-PRWeb/ -- Premia Spine, a leader in motion-preserving spinal technologies, is proud to announce that its study, titled "Lumbar Facet Arthroplasty: An Analysis Comparing Two-Year Outcomes from a Prospective Randomized Clinical Trial Among Patients with Unstable vs. Stable Spondylolisthesis," has been awarded the Best Paper Award at … [Read more...] about Premia Spine Wins Best Paper Award at SMISS 2024 for Study on Lumbar Facet Arthroplasty and the TOPS™ System
NEWS
Stryker announced the addition of two new products recently acquired from 4WEB Medical
Stryker (NYSE: SYK), a global leader in medical technology, has announced the expansion of its Foot & Ankle portfolio with the addition of two new products – the Osteotomy Truss System™ (OTS) and the Ankle Truss System™ (ATS). These products were recently acquired from 4WEB Medical. Stryker will showcase the OTS and ATS, along with the newly acquired Artelon portfolio and … [Read more...] about Stryker announced the addition of two new products recently acquired from 4WEB Medical
Zavation Medical Products, LLC announces launch of Varisync® ALIF System
FLOWOOD, Miss., Sept. 10, 2024 /PRNewswire/ -- Zavation Medical Products, LLC ("Zavation"), an innovator in spinal device technology, proudly announces the launch of its latest product—the Varisync® ALIF System. Engineered to streamline ALIF procedures, the Varisync system offers customization and efficiency with two spacer fixation options: anchors and … [Read more...] about Zavation Medical Products, LLC announces launch of Varisync® ALIF System
Discure Technologies Announces $16 Million in Financing Led by BOLD Capital Partners, Supernova Invest and Sanara Capital
PETACH-TIKVA, Israel, Sept. 10, 2024 /PRNewswire/ -- Discure Technologies Ltd., developer of the Discure System, a bioelectronic implantable device for the treatment of degenerative disc disease (DDD), announced today that the Company has secured $11 million in an oversubscribed Series A financing round, following a SAFE (Simple Agreement for … [Read more...] about Discure Technologies Announces $16 Million in Financing Led by BOLD Capital Partners, Supernova Invest and Sanara Capital
Proprio Successfully Completes 50 Surgeries Using First AI-Powered Surgical Platform; Unveils Massive Dataset
SEATTLE, Sept. 10, 2024 /PRNewswire/ -- Proprio, the global leader in AI-powered surgical technology, announced today that its innovative surgical guidance platform, Paradigm, has reached a significant milestone with 50 successful surgeries completed. These procedures were performed by Dr. Richard Bransford, a world-renowned leader in spine surgery and … [Read more...] about Proprio Successfully Completes 50 Surgeries Using First AI-Powered Surgical Platform; Unveils Massive Dataset
Eminent Spine Scoliosis Deformity Pedicle Screw System Received FDA 510(k) Clearance on August 20, 2024
Plano, TX, September 06, 2024 --(PR.com)-- The Eminent Spine Scoliosis Deformity Pedicle System consists of rods, polyaxial screws with set caps, and cross connectors with locking screws. Additionally, the system consists of rod connectors and iliac bolts with their respective locking screws. Rods are 5.5mm in diameter and are available either straight or pre-contoured. … [Read more...] about Eminent Spine Scoliosis Deformity Pedicle Screw System Received FDA 510(k) Clearance on August 20, 2024
Aurora Spine Corporation to Attend Latin America Pain Society (LAPS) Conference, Showcasing Innovations in Pain Management and Spine Care for Emerging Markets
CARLSBAD, Calif., Sept. 05, 2024 (GLOBE NEWSWIRE) -- Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced its participation in the upcoming Latin America Pain Society (LAPS) Annual Congress September 5-7, 2024, in Mexico City. The … [Read more...] about Aurora Spine Corporation to Attend Latin America Pain Society (LAPS) Conference, Showcasing Innovations in Pain Management and Spine Care for Emerging Markets
SpineGuard obtains EC-MDR certification and launches its Threaded PediGuard for anterior spine surgery in Europe
PARIS (France), BOULDER (CO, USA), September 3, 2024, - 8:00 am CEST - SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, announced today the obtention of the EC-MDR certification for its class IIa and Ir devices, and that it can … [Read more...] about SpineGuard obtains EC-MDR certification and launches its Threaded PediGuard for anterior spine surgery in Europe
Introducing: RealSpine Basic Endo
LEIPZIG, GERMANY, September 02, 2024– Realists Training Technologies GmbH is thrilled to announce the unveiling of RealSpine Basic Endo, a state-of-the-art training simulator designed for multiple endoscopic decompression training of the lumbar spine. The RealSpine Basic Endo offers surgeons a risk-free environment to hone their skills, enhancing both competency and … [Read more...] about Introducing: RealSpine Basic Endo
THINK Surgical Receives FDA 510(k) Clearance for Medacta GMK Sphere® and SpheriKA® implants on TMINI® Miniature Robotic System
FREMONT, Calif., Aug. 29, 2024 /PRNewswire/ -- THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with the GMK® Sphere and SpheriKA® Knee Systems from Medacta International … [Read more...] about THINK Surgical Receives FDA 510(k) Clearance for Medacta GMK Sphere® and SpheriKA® implants on TMINI® Miniature Robotic System
LEM Surgical Celebrates Grand Opening of New Headquarters in Bern
BERN, SWITZERLAND, August 29, 2024 /EINPresswire.com/ -- LEM Surgical AG, a leader in surgical robotics, proudly announces the opening of its new headquarters in Bern, marking a major milestone in the company’s growth and commitment to advancing the field of hard-tissue surgical robotics. The 1800-square-meter facility is designed to foster innovation, streamline operations, … [Read more...] about LEM Surgical Celebrates Grand Opening of New Headquarters in Bern
Quadrant and Evolution Surgical Partner to Accelerate Growth
Quadrant Growth Fund 2 (“Quadrant”) has partnered with Evolution Surgical, an innovative and leading Australian designer and manufacturer of spinal implants. Quadrant is investing and partnering alongside CEO Jack Lancaster and the broader management team. Evolution Surgical is an innovative and leading Australian designer and manufacturer of spinal surgery technology. The … [Read more...] about Quadrant and Evolution Surgical Partner to Accelerate Growth
Spine Wave Announces the Commercial Launch of the Exceed® Biplanar Expandable Interbody Device featuring TiCell® Nano Advanced Surface Technology
SHELTON, Conn., Aug. 26, 2024 (GLOBE NEWSWIRE) -- Spine Wave is pleased to announce the immediate launch of the Exceed® Biplanar Expandable Interbody Device featuring TiCell® Nano Advanced Surface Technology. The Exceed® Biplanar Expandable Interbody Device is an interbody device for Transforaminal (TLIF) and Posterior (PLIF) lumbar interbody fusion with all-titanium … [Read more...] about Spine Wave Announces the Commercial Launch of the Exceed® Biplanar Expandable Interbody Device featuring TiCell® Nano Advanced Surface Technology
SurGenTec Receives FDA Clearance for B-MAN Bone Marrow Aspirate Kit with Integrated Filtration
BOCA RATON, Fla.--(BUSINESS WIRE)--SurGenTec, an innovative medical device company specializing in orthopedic and spine technology, is thrilled to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its proprietary B-MAN Bone Marrow Aspirate Kit. The B-MAN Kit introduces a cutting-edge approach to bone marrow aspiration, … [Read more...] about SurGenTec Receives FDA Clearance for B-MAN Bone Marrow Aspirate Kit with Integrated Filtration
Globus Medical Launches ADIRA™ XLIF™ Plate System
AUDUBON, Pa., Aug. 21, 2024 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced the commercial launch of the ADIRA™ XLIF™ Plate System, the Company’s first product launch compatible across its expanded lateral interbody portfolio. “This launch represents an important milestone in our integration roadmap,” said … [Read more...] about Globus Medical Launches ADIRA™ XLIF™ Plate System
Stryker announces definitive agreement to acquire Vertos Medical, Inc., expanding interventional pain management solutions
Portage, Michigan, Aug. 22, 2024 (GLOBE NEWSWIRE) -- Stryker (NYSE: SYK), a global leader in medical technologies, announced today a definitive agreement to acquire Vertos Medical Inc., a privately held company providing a minimally invasive solution for treating chronic lower back pain caused by lumbar spinal stenosis. Lumbar spinal stenosis affects millions of people … [Read more...] about Stryker announces definitive agreement to acquire Vertos Medical, Inc., expanding interventional pain management solutions
Centinel Spine’s Leadership in Lumbar Total Disc Replacement Fueled Through Rapidly Expanding Market and Long-Term Survivability Data
WEST CHESTER, Pa., Aug. 22, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced data supporting strong market growth … [Read more...] about Centinel Spine’s Leadership in Lumbar Total Disc Replacement Fueled Through Rapidly Expanding Market and Long-Term Survivability Data
Medtronic reports first quarter fiscal 2025 financial results
GALWAY, Ireland, Aug. 20, 2024 /PRNewswire/ -- Medtronic plc (NYSE: MDT) today announced financial results for its first quarter (Q1) of fiscal year 2025 (FY25), which ended July 26, 2024. Key Highlights Financial ResultsMedtronic reported Q1 worldwide revenue of $7.915 billion and adjusted revenue of $8.004 billion, an increase of 2.8% as reported and … [Read more...] about Medtronic reports first quarter fiscal 2025 financial results
restor3d Expands into Robotics and Navigation with Strategic Leadership Hires
DURHAM, N.C.--(BUSINESS WIRE)--restor3d, the medical technology leader in 3D printed, personalized orthopedic implants, announced its strategic expansion into the field of robotics and navigation through the appointment of seasoned leaders, Larry Hazbun and Kirstin Widding. restor3d is at the forefront of innovation in the field of personalized orthopedic solutions, … [Read more...] about restor3d Expands into Robotics and Navigation with Strategic Leadership Hires