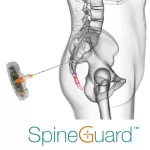
PARIS, and BOULDER (CO), February 10, 2025 - 6:00 pm CET – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announces that its commercial partner Omnia Medical launched their codeveloped product PsiFGuard in the USA … [Read more...] about SpineGuard announces the commercial launch of its “PsiFGuard” new smart drilling device dedicated to sacroiliac joint fusion
NEWS
We are proud to announce that Genesys Spine will once again sponsor SPINEMarketGroup in 2025!
Thank you, Genesys Spine! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About Genesys Spine Founded in December of 2009, Genesys Spine’s mission was to bring a suite of medical implants and instruments with novel characteristics … [Read more...] about We are proud to announce that Genesys Spine will once again sponsor SPINEMarketGroup in 2025!
Sunday Reflections on the Changes in the Spine Market!
As we move into February, it's remarkable how quickly things are evolving in the Spine market. In just over a month, we have been confronted with two significant pieces of news involving major competitors: Stryker and Globus Medical. As many of you know, Stryker sold its Spine business to V.B (Viscogliosi Brothers) just two weeks ago, and more recently, Globus Medical acquired … [Read more...] about Sunday Reflections on the Changes in the Spine Market!
Johnson & Johnson MedTech Secures Exclusive Distribution Deal with CG Bio for NOVOSIS in Asia
Johnson & Johnson MedTech (J&J MedTech), a leading player in medical technology, has entered into an exclusive distribution and promotional agreement with CG Bio and its subsidiary CG MedTech for the commercialization of NOVOSIS, an advanced bone graft product. The partnership aims to improve surgical outcomes in trauma and spinal procedures by broadening access to … [Read more...] about Johnson & Johnson MedTech Secures Exclusive Distribution Deal with CG Bio for NOVOSIS in Asia
Paradigm Spine GmbH and RIWOspine UK are pleased to announce the strategic partnership for the UK distribution of the eFuse® expandable interbody cages with surface technology.
Paradigm Spine GmbH, a leading innovator in spinal technologies, is pleased to announce a strategic partnership with RIWOspine UK to distribute its cutting-edge eFuse® expandable interbody cages with surface technology across the United Kingdom. This collaboration marks a significant step forward in advancing minimally invasive spine surgery techniques, particularly for … [Read more...] about Paradigm Spine GmbH and RIWOspine UK are pleased to announce the strategic partnership for the UK distribution of the eFuse® expandable interbody cages with surface technology.
What Are the Strategic Reasons Behind Globus Medical’s Acquisition of Nevro Corp.?
Globus Medical’s acquisition of Nevro Corp. is more than just a business deal. It’s a bold move that could reshape the company’s direction and make a real difference in how chronic pain is managed. So, what’s behind this acquisition? Let’s dive into the key reasons and see why it could be a game-changer for both the company and the people who need better pain … [Read more...] about What Are the Strategic Reasons Behind Globus Medical’s Acquisition of Nevro Corp.?
Globus Medical to Acquire Nevro Corp. to Expand Treatment Options for Patients
AUDUBON, Pa. and REDWOOD CITY, Calif., Feb. 06, 2025 (GLOBE NEWSWIRE) -- Globus Medical (NYSE: GMED), a leading musculoskeletal solutions company, and Nevro Corp. (NYSE: NVRO), a global medical device company that is delivering comprehensive, life-changing solutions for the treatment of chronic pain, today announced they have entered into … [Read more...] about Globus Medical to Acquire Nevro Corp. to Expand Treatment Options for Patients
We are proud to announce that Eminent Spine will once again sponsor SPINEMarketGroup in 2025!
Thank you, Eminent Spine! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About Eminent Spine Forged in solid biomechanics and the best-known biomaterials, Eminent Spine has emerged as an industry leader in the evolution of spinal … [Read more...] about We are proud to announce that Eminent Spine will once again sponsor SPINEMarketGroup in 2025!
Aclarion Announces CLARITY Trial for Nociscan Now Fully Funded
BROOMFIELD, Colo., Feb. 04, 2025 (GLOBE NEWSWIRE) -- Aclarion, Inc., (“Aclarion” or the “Company”) (Nasdaq: ACON, ACONW), a healthcare technology company that is leveraging biomarkers and proprietary augmented intelligence (AI) algorithms to help physicians identify the location of chronic low back pain, announced today that it has secured full funding for its pivotal CLARITY … [Read more...] about Aclarion Announces CLARITY Trial for Nociscan Now Fully Funded
We are proud to announce that Normmed will once again sponsor SPINEMarketGroup in 2025!
Thank you, Normmed! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About Normmed Normmed makes a difference in both domestic and international markets with innovative solutions in its field and progresses on this path. Normmed, which … [Read more...] about We are proud to announce that Normmed will once again sponsor SPINEMarketGroup in 2025!
We are proud to announce that Z-Medical will once again sponsor SPINEMarketGroup in 2025!
Thank you, Z-Medical! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About Z-Medical GmbH + Co. KG Based and founded in Tuttlingen in 2010, Z-Medical GmbH + Co. KG is a privately financed and held medical device company that designs, … [Read more...] about We are proud to announce that Z-Medical will once again sponsor SPINEMarketGroup in 2025!
(UPDATED 2025): +15 Fixation Bands to Know..!
Sublaminar band fixation is a surgical technique commonly used for the treatment of spinal deformities, such as scoliosis. It involves placing small, flexible, and durable bands, typically made of stainless steel or polyester, around the spinal processes. These bands are then tightened to correct spinal curvature and maintain the corrected alignment.This technique is often used … [Read more...] about (UPDATED 2025): +15 Fixation Bands to Know..!
Life Spine Announces FDA 510(k) Clearance for the ProLift® Pivot Expandable Spacer System
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, Inc., a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the ProLift Pivot Expandable Spacer System. The ProLift Pivot System represents the … [Read more...] about Life Spine Announces FDA 510(k) Clearance for the ProLift® Pivot Expandable Spacer System
Globus Medical Launches HILINE™ System: Innovation in Posterior Spinal Fixation
Globus Medical, a leader in medical device technology for the spine, has launched the new HILINE™ system, an innovative posterior band fixation system for the cervical and thoracolumbar spine. Designed with robust implants and advanced instrumentation, HILINE™ aims to optimize reduction in scoliosis correction, provide reliable stabilization in compromised anatomy, and … [Read more...] about Globus Medical Launches HILINE™ System: Innovation in Posterior Spinal Fixation
Stryker reports 2024 operating results and 2025 outlook
Portage, Michigan, Jan. 28, 2025 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) reported operating results for the fourth quarter and full year of 2024: Fourth Quarter Results Full Year Results “We delivered another year of double-digit organic sales growth while continuing to expand adjusted operating margins and drive adjusted earnings per share growth,” said Kevin … [Read more...] about Stryker reports 2024 operating results and 2025 outlook
We are proud to announce that GLOBAL biomedica will once again sponsor SPINEMarketGroup in 2025!
Thank you, GLOBAL biomedica! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About GLOBAL biomedica GLOBAL biomedica is manufacturer of orthopedic titanium implants, extended by designing and constructing in collaboration with leading … [Read more...] about We are proud to announce that GLOBAL biomedica will once again sponsor SPINEMarketGroup in 2025!
Stryker’s Spine Business Sale: A Smart Move or a Missed Opportunity?
Stryker’s recent decision to divest its spinal implant business marks a significant shift in the medical device industry. This move allows the company to focus on higher-growth, more profitable segments like robotics and vascular medicine. However, it also will enable competitors to strengthen their position in the spine market. For VB Spine, success will depend on execution: … [Read more...] about Stryker’s Spine Business Sale: A Smart Move or a Missed Opportunity?
Stryker announces definitive agreement for the sale of its U.S. spinal implants business and plans to sell related international business
Portage, Michigan, Jan. 28, 2025 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK), a global leader in medical technologies, announced today a definitive agreement to sell its U.S. spinal implants business to Viscogliosi Brothers, LLC, a family-owned investment firm specializing in the neuro-musculoskeletal space, to create a newly formed company called VB Spine, LLC. “We believe that … [Read more...] about Stryker announces definitive agreement for the sale of its U.S. spinal implants business and plans to sell related international business
We are proud to announce that SpineGuard will once again sponsor SPINEMarketGroup in 2025!
Thank you, SpineGuard! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a GOLD sponsor for 2025.We are very proud to continue this journey together for another year! About SpineGuard Pierre Jérôme and Stéphane Bette founded SpineGuard in January 2009 with the goal of delivering the clinical benefits of the DSG™ (Dynamic Surgical … [Read more...] about We are proud to announce that SpineGuard will once again sponsor SPINEMarketGroup in 2025!