DALLAS & LYON, France--(BUSINESS WIRE)--Regulatory News: SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris, ISIN: FR0014005I80 / Ticker: ALSMA), a French-American player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today announces … [Read more...] about SMAIO Signs a New Distribution Agreement for Its Open Platform KEOPS-4ME With Orthofix in the U.S.
NEWS
NGMedical partners with Spanish company Cardiva to distribute the MOVE®-c cervical disc prosthesis
This technology offers spine surgeons a practical and easy-to-implement solution for their patients NONNWEILER, SAARLAND, GERMANY, October 20, 2025 /EINPresswire.com/ -- NGMedical, a leader in innovation in spinal implants, announces a new strategic agreement with Cardiva's Traumatology Division for Spain. This collaboration includes the distribution and marketing of the … [Read more...] about NGMedical partners with Spanish company Cardiva to distribute the MOVE®-c cervical disc prosthesis
Worldwide First: Biedermann Motech Further Expands its MOSS! MODULARITY Platform and Announces Introduction of Multiple New Products
SCHWENNINGEN, Germany--(BUSINESS WIRE)--Biedermann Motech, the prominent innovator in next generation spinal and extremity implant systems and procedural solutions, today announced the market introduction of several additions to its MOSS!MODULARITY™ platform. MOSS!MODULARITY encompasses a broad range of products and technologies for spinal surgery based around the concept of … [Read more...] about Worldwide First: Biedermann Motech Further Expands its MOSS! MODULARITY Platform and Announces Introduction of Multiple New Products
China’s Spinal Implant Market: Do You Know the Essentials? What Strategies Should Foreign Companies Consider?
How is the Chinese Spinal Implant Market Growing? The Chinese spinal implant market is entering a phase of accelerated growth. According to Grand View Research, the market for specific spinal implants reached approximately US$450.6 million in 2024 and is projected to grow to US$929.3 million by 2033, with a CAGR of 8.5% between 2025 and 2033. When including devices for … [Read more...] about China’s Spinal Implant Market: Do You Know the Essentials? What Strategies Should Foreign Companies Consider?
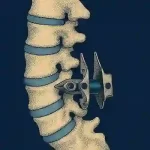
The Facet Cage: The Missing Link in Spinal Fusion? Could this new technology redefine posterior spinal instrumentation?
Spinal surgery has long sought the optimal strategy for achieving stable fusion between adjacent vertebrae. Traditionally, posterior fixation has relied on pedicle screws, rods, and interbody cages. However, stabilization of the facet joint—until recently an underutilized site for direct fusion—has attracted growing attention as technology and biomechanics have … [Read more...] about The Facet Cage: The Missing Link in Spinal Fusion? Could this new technology redefine posterior spinal instrumentation?
NGMedical will present its unique Product Portfolio at Eurospine
NGMedical GmbH, a leading innovator in cervical disc prosthetic technology, is excited to present its innovative product portfolio at Eurospine in Copenhagen. NONNWEILER, GERMANY, October 16, 2025 /EINPresswire.com/ -- NGMedical GmbH, a leading innovator in cervical disc prosthetic technology, is excited to present its innovative product portfolio at Eurospine in … [Read more...] about NGMedical will present its unique Product Portfolio at Eurospine
FFX® Becomes the First Facet Cage Cleared by the FDA for Standalone Use.
Strasbourg, France – October 16, 2025 – SC Medica today announced that its FFX® Facet FiXation System has received U.S. Food and Drug Administration (FDA) clearance for standalone use, making it the first and only facet fusion cage approved for lumbar spine surgery without supplemental fixation. Following its initial FDA clearance in … [Read more...] about FFX® Becomes the First Facet Cage Cleared by the FDA for Standalone Use.
Legacy Spine Companies Retreat While KIC Ventures Builds New Markets in Outpatient Interventional Spine Surgery, Regenerative Biologics, and Viscoelastic Disc Replacements
FORT LAUDERDALE, Fla., Oct. 15, 2025 /PRNewswire/ -- As legacy spine and orthopedic companies divest from their core device portfolios and retreat from innovation, Harvard-trained orthopedic spine surgeon and CEO Dr. Kingsley R. Chin has demonstrated consistent leadership in guiding KIC Ventures to advance a bold new vision — building sustainable … [Read more...] about Legacy Spine Companies Retreat While KIC Ventures Builds New Markets in Outpatient Interventional Spine Surgery, Regenerative Biologics, and Viscoelastic Disc Replacements
Do Interspinous Spacers Still Have a Place in Spine Surgery?
In the mid-2000s, interspinous devices emerged in spine surgery as a promising new approach. Placed between the lumbar spinous processes, they aimed to relieve spinal stenosis or chronic low back pain through indirect decompression of the spinal canal, without the need for fusion or rigid instrumentation. I recall that at the time, they were considered truly successful, almost … [Read more...] about Do Interspinous Spacers Still Have a Place in Spine Surgery?
EXALTA Secures EU-MDR Certification for Titanium Compression Screws, Expanding Access to Advanced Trauma Solutions
BETHLEHEM, PA, UNITED STATES, October 15, 2025 /EINPresswire.com/ -- EXALTA Group, a global leader in the development and manufacturing of integrated OEM solutions for mission-critical medical devices, today announced it has obtained European Medical Device Regulation (EU-MDR) certification for its full range of headed and headless titanium compression screws used in trauma and … [Read more...] about EXALTA Secures EU-MDR Certification for Titanium Compression Screws, Expanding Access to Advanced Trauma Solutions
Amnovis Celebrates 5 Years of Innovation with 100,000 3D-Printed Implants Shipped
Aarschot, Belgium – October14, 2025. Amnovis, a contract manufacturer specializing in 3D-printed medical devices and high-performance applications, proudly marks its fifth anniversary with the shipment of its 100,000th 3D-printed implant. Founded in June 2020 by Peter Mercelis and Ruben Wauthle, Amnovis was built on a clear mission: to deliver high-quality products, … [Read more...] about Amnovis Celebrates 5 Years of Innovation with 100,000 3D-Printed Implants Shipped
Centinel Spine® Receives Two-Level FDA Approval for prodisc® C Vivo and prodisc® C SK Match-the-Disc™ Cervical Total Disc Replacement Devices
WEST CHESTER, Pa., Oct. 14, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced U.S. Food and Drug Administration … [Read more...] about Centinel Spine® Receives Two-Level FDA Approval for prodisc® C Vivo and prodisc® C SK Match-the-Disc™ Cervical Total Disc Replacement Devices
SMAIO Announces Strong First-Half 2025 Results and Robust Growth in Business Activity in the Third Quarter of 2025
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris, ISIN: FR0014005I80 / Ticker : ALSMA), a French-American player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today publishes its consolidated results for the first half of 20251 and its … [Read more...] about SMAIO Announces Strong First-Half 2025 Results and Robust Growth in Business Activity in the Third Quarter of 2025
Johnson & Johnson Follows in Zimmer Biomet’s Footsteps: An Analysis of the DePuy Synthes Spin-Off Compared to ZimVie
Johnson & Johnson (J&J) announced today its plan to spin off its orthopaedics business, which will operate as an independent company under the name DePuy Synthes. This move echoes a similar strategy by Zimmer Biomet in 2022, when it separated its spine and dental units to form ZimVie. Both cases highlight a growing trend in the MedTech sector: streamlining conglomerates … [Read more...] about Johnson & Johnson Follows in Zimmer Biomet’s Footsteps: An Analysis of the DePuy Synthes Spin-Off Compared to ZimVie
Johnson & Johnson Announces Intent to Separate Its Orthopaedics Business
Strengthens focus of Johnson & Johnson as an innovation powerhouse and accelerates the portfolio shift of its MedTech Segment to higher-growth and higher-margin markets Standalone orthopaedics business would operate as DePuy Synthes and be the largest, most comprehensive orthopaedics-focused company in the world Namal Nawana appointed to serve as Worldwide … [Read more...] about Johnson & Johnson Announces Intent to Separate Its Orthopaedics Business
From Early Innovations to Modern Practice: Where Are Dynamic and Semi-Rigid Spine Systems Today?
Following the recent publication this week of the MDR for the BDyn dynamic rod, I felt it was timely to revisit a topic that had a significant impact in the early 2000s, when dynamic stabilization systems experienced a major surge in interest and adoption. This development reminds us of the clinical and technological debates surrounding motion-preserving spinal implants, … [Read more...] about From Early Innovations to Modern Practice: Where Are Dynamic and Semi-Rigid Spine Systems Today?
Next-Generation AI-Driven SyncAR® Spine Receives FDA Clearance for Spine Surgery
By bringing MRI and CT directly into the operating room, SyncAR Spine turns preoperative planning imaging into real-time intraoperative guidance. CLEVELAND, Oct. 10, 2025 /PRNewswire/ -- Surgical Theater, the leader in surgical XR visualization, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for SyncAR® Spine, the next-generation … [Read more...] about Next-Generation AI-Driven SyncAR® Spine Receives FDA Clearance for Spine Surgery
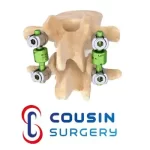
BDyn Posterior Dynamic Stabilization System Earns MDR Certification!
Cousin Surgery is proud to announce a major milestone: BDyn Posterior Dynamic Stabilization System has officially received MDR (Medical Device Regulation – EU 2017/745) certification! This achievement marks an important step forward in our ongoing commitment to the highest European standards of safety, quality, and performance for medical devices. Raising the bar for … [Read more...] about BDyn Posterior Dynamic Stabilization System Earns MDR Certification!
SpineGuard reports its Q3 2025 revenue
PARIS and BOULDER (CO), October 9, 2025 – 6:00 pm CEST - SpineGuard (FR0011464452 - ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announces its third quarter 2025 revenue. Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, … [Read more...] about SpineGuard reports its Q3 2025 revenue