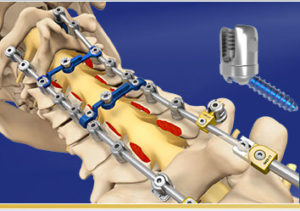
SHELTON, Conn., Feb. 21, 2018 (GLOBE NEWSWIRE) — Spine Wave is increasing the Proficient® Posterior Cervical Spine System’s product offering to further serve the posterior cervical spine market. The system is gaining rapid adoption by spine surgeons, primarily due to its patented tri-lobe polyaxial screw design which offers a best in class 120 degrees of […]
NEWS
AxioMed Appoints Disc Replacement and Total Motion Expert Winni Koenig as VP Strategic Markets
MALDEN, MASS. (PRWEB) FEBRUARY 20, 2018–AxioMed announced today its latest move to expand its executive team internationally with the appointment of Winni Koenig as Vice President Strategic Markets. The company recently completed their lumbar U.S. IDE and will soon commence large scale manufacturing of their cervical and lumbar disc at their new Boston facility, positioning […]
NuVasive files alleged poaching, infringement suits against Alphatec
(Massdevice.com)–NuVasive Inc. (NSDQ:NUVA) has filed a suit against Alphatec (NSDQ:ATEC) claiming the company hired on executives, including former COO and prez Pat Miles, with knowledge of its XLIF lateral interbody fusion procedures in an attempt to recreate the technology for its own products. In its infringement suit, submitted this week to the U.S. District Court […]
Nexxt Spine Marks 250th Implantation of the NEXXT MATRIXX® System Interbody
NOBLESVILLE, Ind.–(BUSINESS WIRE)–Nexxt Spine, LLC, a medical device company focused on designing, manufacturing, and distributing innovative spinal solutions, today announced the milestone of the 250th implantation of the NEXXT MATRIXX® System. Launched in 2017 the NEXXT MATRIXX® System is a collection of 3D printed porous titanium interbodies that leverages Nexxt generation technology to create interbody and […]
Mazor CEO: Robotics is Here to Stay
(https://www.mddionline.com)–During Mazor Robotics’ fourth-quarter earnings call, executives mentioned Medtronic almost as much as they mentioned their own company. And for good reason. The company received 24 purchase orders for its Mazor X robotic system in the fourth quarter of 2017, and all but one of those orders came from Medtronic. Under a global distribution sale that […]
SpineGuard and Adin Dental Implant Systems Announce the Successful Completion of a Study Demonstrating the Benefits of Dsg™ Technology for Dental Implant Placement
PARIS & SAN FRANCISCO–(BUSINESS WIRE)–Regulatory News:SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that develops and markets instruments designed to secure the placement of surgical implants, has announced together with Adin Dental Implant Systems and its subsidiary, Confident Ltd., the successful completion of a key milestone in the validation of prototypes for dental surgery, results […]
Stryker Crosses The 95% Thresholds in VEXIM and Announces Its Intention to File a Proposed Public Buy-out Offer Followed by a Squeeze-out for the Outstanding Shares of VEXIM
BALMA, France–(BUSINESS WIRE)–Regulatory News: Stryker Corporation (NYSE:SYK) announced that it has crossed the 95% thresholds in VEXIM (Paris:ALVXM) (FR0011072602 – ALVXM) and intends to file a proposed public buy-out offer followed by a squeeze-out for the outstanding shares of VEXIM, in accordance with French tender offer laws and regulations. After the acquisition of 92.19% of the […]
Spineology Completes Enrollment in IDE Trial for Mesh Fusion Implant
ST. PAUL, Minn.–(BUSINESS WIRE)–Spineology Inc., an innovator in anatomy-conserving spine surgery, is excited to announce that enrollment is now complete in the Company’s SCOUT clinical trial. The SCOUT (Spineology Clinical Outcomes Trial) IDE, conducted under an FDA-approved protocol, is a prospective multicenter non-randomized performance goal investigation, designed to evaluate safety and effectiveness outcomes in instrumented […]
Simplify Medical Completes Enrollment in IDE Trial Studying Simplify Disc in One Level of Spine for Cervical Disc Replacement
SUNNYVALE, Calif.–(BUSINESS WIRE)–Simplify Medical Pty Ltd, maker of the Simplify® cervical artificial disc, today announced completion of enrollment in its U.S. IDE trial studying the Simplify Disc used for one-level cervical implantation of the device between C3 to C7 compared with a historical control group. The trial enrolled 166 patients at 16 U.S. sites. “We […]
TranS1® Announces Three New Patents
Denver, Feb. 13, 2018 (GLOBE NEWSWIRE) — TranS1®, a spinal device company focused on breakthrough solutions that minimize tissue trauma, today announced it has added three new patents to its portfolio. The patents relate to two new developments associated with presacral interbody fusion, including two new expandable cages designed to create lordosis at L5-S1, along […]
EIT Emerging Implant Technologies Announces CE Mark and First Cases for the World’s First Fully 3D Printed Adjustable Cage
TUTTLINGEN, GERMANY (PRWEB) FEBRUARY 13, 2018–Emerging Implant Technologies GmbH (EIT), a German medical device manufacturer exclusively focused on creating innovative technologies for spinal application by utilizing additive manufacturing, announces that it has received CE mark and performed the first surgeries in Germany with the first fully 3D printed adjustable interbody fusion cage worldwide. The adjustable EIT […]
Camber Spine Technologies Announces First Implantations Of SPIRA™-C Open Matrix Cervical Interbody
WAYNE, Pa., Feb. 12, 2018 /PRNewswire/ — Camber Spine, a leading innovator in spine and medical technologies, today announced the first surgeries using the company’s proprietary SPIRA™-C Open Matrix Cervical Interbody device, a unique, interbody fusion implant consisting of spiral support arches and Surface by Design™ technology. The first cases were performed successfully this past […]
Simplify Medical Closes Additional $23.25 Million in Second Tranche of Series B Financing Totaling $44.25 Million
SUNNYVALE, Calif. – February 9, 2018 – Simplify Medical Pty Ltd, maker of the Simplify® cervical artificial disc, today announced a second tranche of its Series B financing of $23.25 million, completing the oversubscribed round totaling $44.25 million. The lead investor for the second tranche is LSP Health Economics Fund 2 (LSP HEF 2), with participation from existing venture investors […]
John DeFord To Join NuVasive Board Of Directors
SAN DIEGO, Feb. 8, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, announced the appointment of John A. DeFord, Ph.D. to the Company’s Board of Directors, effective upon the filing of the Company’s Form 10-K with the Securities and Exchange Commission. Dr. DeFord has […]
joimax® Continues Strong Growth Trend in 2017 with Launch of Its MultiZYTE® Facet and SI Joint Treatment Set in the US
KARLSRUHE, Germany–(BUSINESS WIRE)–the joimax® group announces very strong sales figures for 2017. The German based market leader of technologies and training methods for endoscopic minimally invasive spinal surgery shows a jump of almost 80% vs. 2016 in the U.S. Consolidated sales show a growth of nearly 30%, which reflects its CAGR of 35% since 2008. “joimax® is […]
Life Spine to Showcase Micro-Invasive Technologies at ICSJS, AAOS and the AANS/CNS Spine Summit
HUNTLEY, Ill.–(BUSINESS WIRE)–Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today its 2018 participation at ICSJS, AAOS and the AANS/CNS Spine Summit. The meetings are expected to have a combined attendance of over 13,000 medical attendees. “These industry meetings are pivotal in […]
Orthofix Announces 510(k) Clearance and US Limited Market Launch of FORZA XP Expandable Spacer System
LEWISVILLE, Texas–(BUSINESS WIRE)–Orthofix International N.V., (NASDAQ: OFIX), a global medical device company focused on musculoskeletal healing products, today announced the 510(k) clearance and U.S. limited market launch of the FORZA® XP Expandable Spacer System. Designed to restore normal disc height in patients suffering from degenerative disc disease, the FORZA XP Expandable Spacer System can be […]
ChoiceSpine™ Announces Key Executive Leadership Changes
KNOXVILLE, TENN. (PRWEB) FEBRUARY 06, 2018–ChoiceSpine LP, a privately-held spinal device manufacturer based in Knoxville, TN, today announced changes to strengthen its executive leadership team and continue driving the company’s industry-leading product portfolio and financial growth. The two leadership changes are: Stephen Ainsworth, PhD. promoted to Executive Vice President, Strategy and Technology; and Kurt Kessler […]
Aurora Spine Announces Upsizing of Private Placement
CARLSBAD, Calif., Feb. 05, 2018 (GLOBE NEWSWIRE) — Aurora Spine Corporation (TSX-V:ASG) (“Aurora Spine” or the “Company”) is pleased to announce that, as a result of strong investor demand, it has increased the size of its previously announced non-brokered private placement offering of common shares to up to 10,000,000 common shares, at a price of […]
We are proud and happy to announce that FMI Instrumed is Sponsor of SPINEMarketGroup in 2018!
Thank you FMI Instrumed! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About FMI Instrumed FMI Instrumed, is a contract manufacturer of choice for the global medical instruments and implant industries. We specialize in the series production of implants such as cages, […]
Learn about 46 Contract Manufacturers in Spine
The major orthopedic medical device companies have a different outsourcing strategy than more of their smaller challengers. While the large OEMs manufacture a fair amount of product in-house, startups, small and midsize firms outsource almost all of their products. But recently, since OEMs are eager to introduce innovative new products to market as quickly as […]
SeaSpine Expands Ventura™ NanoMetalene® Implant Offering to Accommodate Larger Range of Posterior Procedures
CARLSBAD, Calif., Jan. 31, 2018 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ:SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the portfolio expansion of the Ventura™ NanoMetalene® posterior interbody device. Ventura NanoMetalene, which has been commercially available since 2015, has expanded its size offerings to accommodate […]
We are proud and happy to announce that Z-Medical is Sponsor of SPINEMarketGroup in 2018!
Thank you Z-Medical! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About Z-Medical GmbH + Co. KG Based and founded in Tuttlingen in 2010, Z-Medical GmbH + Co. KG is a privately financed and held medical device company that designs, develops, […]
We are proud and happy to announce that SpineGuard is again Sponsor of SPINEMarketGroup in 2018!
Thank you SpineGuard! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About SpineGuard Pierre Jérôme and Stéphane Bette founded SpineGuard in January 2009 with the goal of delivering the clinical benefits of the DSG™ (Dynamic Surgical Guidance) technology to as many […]
We are proud and happy to announce that iSpine is again Sponsor of SPINEMarketGroup in 2018!
Thank you iSpine! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About iSpine iSpine is a South African company that was formed to meet the needs of Neuro and Spinal surgeons the world over.iSpine and its expert partners, designs, develops and […]
We are proud and happy to announce that SPINEART is again Sponsor of SPINEMarketGroup in 2018!
Thank you SPINEART! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About Spineart Spineart is a privately held medical device company focused on simplifying the surgical act by designing, developing and promoting safe and efficient solutions to spine surgeons, operating room […]
We are proud and happy to announce that BIOMECH is again Sponsor of SPINEMarketGroup in 2018!
Thank you BIOMECH! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2018. About Biomech Biomech is one the best orthopedic company in Taiwan for 27 years, focusing on designing/manufacturing spinal devices and instruments. Our goal is to decrease surgical process for doctors […]