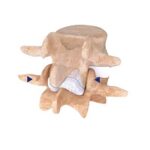
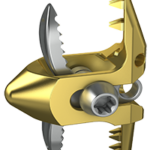
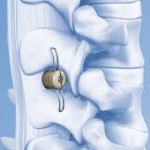
Evo is an innovative interlaminar fusion device made entirely with 3D printing technology, composed of a macrostructure in trabecular titanium that promotes rapid bone growth. Its central body in trabecular titanium – perforated both in the craniocaudal and lateral direction – allows the surgeon to insert a greater quantity of bone graft which increases the reachable area of … [Read more...] about Evo
Interspinous Devices
Ellipse
Ellipse is a minimally invasive interspinous spacer which characteristics are very innovative: its components are made of two materials with different peculiarities, PEEK and titanium, thus combining the advantages of both so as to obtain a highly innovative result.The functional part of the device which is subject to load forces is made of PEEK, a new generation plastic … [Read more...] about Ellipse
Fixpino
The Fixpino, interspinous fixation system, is designed to insert the cross-sectional diameter of the stenotic canal in patients suffering from neurogenic claudication. The Fixpino is an S-shaped metallic device that is inserted between the spinous processes. The S-shaped spacer fits between the spinous processes and the wings are designed to prevent the implant from moving. … [Read more...] about Fixpino
Falena
The Falena is an interspinous spacer indicated for surgical decompression in cases with spinal and radicular stenosis performed with a minimally invasive approach.The device is composed of two components: a winged structure, formed by a main pin with two wings at the extremities and a cap to allow for an atraumatic crossing of the interspinous ligament a “C” spring … [Read more...] about Falena
FLEXIS
The FLEXIS interspinous rotating device is used for the surgical treatment of spinal pathologies. This treatment consists in distracting two or more vertebrae among them, and provide a stabilization. This distraction is made between the two spinous processes of two adjacent vertebrae. For best results, a detailed preoperative evaluation, a meticulous surgical technique and … [Read more...] about FLEXIS
Gelfix
The GelFix™ Interspinous Spacer is a one piece posterior spinal distraction implant made from RMI’s biocompatible hydrogel. Implanted between the spinous process through a small incision, GelFix™ acts as a dynamic spacer restricting painful motion without adversely affecting other segmental motion. GelFix™ increases space between the spinous processes in order to maintain … [Read more...] about Gelfix
HUVEX
HUVEX is a single-level, posterior, non-pedicle supplemental fixation device intended for use in the lumbar spine (L1~S1) as an adjunct to fusion in skeletally mature patients. It is intended for fixation/attachment to the spinous process for the purpose of achieving supplemental fusion in the following conditions : degenerative disc disease , spondylolisthesis, trauma (i.e., … [Read more...] about HUVEX
Helifix
HeliFix Interspinous Spacer System features a self-distracting PEEK implant with simplified and straightforward instrumentation. The percutaneous delivery of the implant enables surgeons to preserve the surrounding anatomical structures - lessening the overall surgical trauma to the aging population. The HeliFix System is intended for patients suffering from lumbar spinal … [Read more...] about Helifix
HEKTOR
HEKTOR is an optima peek implant that is placed between two spinous processes. When implanted, the HEKTOR is not positioned close to nerves or the spinal cord, but rather behind the spinal cord, between the spinous processes. HEKTOR VIDEO ANIMATION HEKTOR-Brochure.BMK.pdf Benefits: Anatomical shape Minimally invasive procedure with simple steps Preservation of … [Read more...] about HEKTOR
iFIX® (Interspinous Fusion System)
iFIX® is an Interspinous Fusion System manufactured by Seohancare. Features Graft Window for maximizing fusion rate Motional Spikes for optimal fixation and fusion Full polyaxial design Easy locking mechanism About Interspinous Devices Interspinous spacers sometimes also called as Interspinous process decompression systems, are the devices implanted between … [Read more...] about iFIX® (Interspinous Fusion System)
iSPACE
Elite’s Spine division offers a wide range of products that are shaped by the philosophy: premium quality products with ease of use instrumentation. From simple products like PEEK spacers to total cervical disc replacements and expandable corpectomy cages and accompanying instrumentation. Elite strives to continually to expand its product line with devices and biologics that … [Read more...] about iSPACE
ip|spine
The idea of this dynamic interspinous process device is as unique as are its benefits. ip|spine is probably the most minimally invasive spacer in the area of lumbar implantology. The neccesity of only one unilateral incision leads to a tissue-conserving approach that leaves the ligamentum flavum untouched and, therefore, significantly reduces risks and recovery time. With this … [Read more...] about ip|spine
IntraSPINE®
IntraSPINE® is a dynamic interlaminar stabilization system indicated to treat skeletally mature patients suffering from pain, numbness, and/or cramping in the legs (neurogenic intermittent claudication) secondary to a diagnosis of DDD from L1 to S1.The device is made of medical dimethyl siloxane and covered with pure polyethylene terephtalate. The frontal extremity is coated … [Read more...] about IntraSPINE®
Inspan VEGA with self locking screw fixation
Inspan was developed by Orthopedic spine surgeons and FDA cleared in 2010 to treat degenerative spine disease with stenosis. It comes in different designs based on the indication, anatomy and patient. Example Inspan Slim for L5-S1. Inspan Vega for spondylolisthesis. Self-locking screws for added fixation as in trauma, Scoliosis or spondylolisthesis.www.MyInspan.com Inspan … [Read more...] about Inspan VEGA with self locking screw fixation
InterBRIDGE®
InterBRIDGE® is a lumbar interspinous fusion implant inserted through unilateral and mini-invasive posterior approach allowing supraspinous ligament sparing. Its insertion and its deployment are realized in one single step in order to minimize operating time. InterBRIDGE VIDEO ANIMATION (LDR Version) Interbridge Brochure.pdf Benefits: Posterior column stabilized … [Read more...] about InterBRIDGE®
IMPALA®
Minor intervention. Major impact As an alternative to fusion, minimally invasive surgery is becoming increasingly important thanks to the considerable protection of tissue it offers. With IMPALA® we offer an implant that pays close attention to the preservation of the surrounding anatomical structures compared to traditional surgical procedures. The three-part IMPALA® … [Read more...] about IMPALA®
Inspan SLIM
Inspan SLIM Spinous Process Plate System’s low profile design gives it a minimal footprint within the patient. Its dual interlocking hub and dual set screws provide a rigid design. Its titanium composition supplies strength for fixation. Inspan VIDEO ANIMATION Inspan-SGT.les-society.pdf Features: Titanium implants provide superior strength and fixation Low profile … [Read more...] about Inspan SLIM
InSWing
InSWing™ Interspinous Spacer is a decompression solution developed to relieve pain from lumbar spinal stenosis (LSS). This device offers the advantages of a reduced incision size, minimized muscle trauma and preservation of the supraspinous ligament.InSWing offers a less invasive surgical approach, requires minimal or local anesthesia, reduces blood loss, and results in shorter … [Read more...] about InSWing
InSpace
In Space is an interspinous spacer, intended to stop segmental extension and to distract the interspinous space e.g. for dynamic lumbar stenosis. It is implanted through a percutaneous, tissue preserving approach. In-Space-SGT.DePuySynthes (Synthes).pdf Benefits: Percutaneous lateral approach;No stripping of the paraspinal muscle Tissue sparing procedure:Stabilizing … [Read more...] about InSpace