The first LfC’s 3D-Frame-Ti implant was the CarЯLIF (Carving Round Lumbar Interbody Fusion) – it is the first 3D printed, curved spinal cage worldwide. The clinical implantation started in the year 2011.This cage combines the fusion enhancing features of the 3D-Truss-Ti structure together with the precision of insertion and placement provided by the unique “carving guides”. It is still the “flagship” implant of the whole CarЯLIF-family – a group of implants based on 3D-Truss-Ti Electron Beam Technology – which has been applied for the first time in spinal implant by LfC.Those implants constitute a new significant step in spine surgery due to their special function enhancing new trend in surgical treatment of the spine:“Bridging of spine” with 3D-Truss-Ti construction
Technology
At the end of the 20th century, technologies stemming from aero-space engineering opened a world of new possibilities in 3D spatial architecture. LfC managed to transmit this space technology to the field of spinal implants. Electron beam melting (EBT-Electron Beam Technology) of Ti-alloy powder with temperatures over 2000 deg. C in a vacuum chamber -that is the essence of technology used to create a new generation of 3D-implants. Apart from the structure itself, which favors bone ingrowth within the spatial cells, the implant’s design enables an accelerated osteointegration mechanism reducing fusion time by 40-50 percent and more. This new phenomenon was studied and called „Ivy-like mechanism, L.C.” as a metaphor for the natural growth process of ivy, which climbs along the specially made rough surface of truss design.
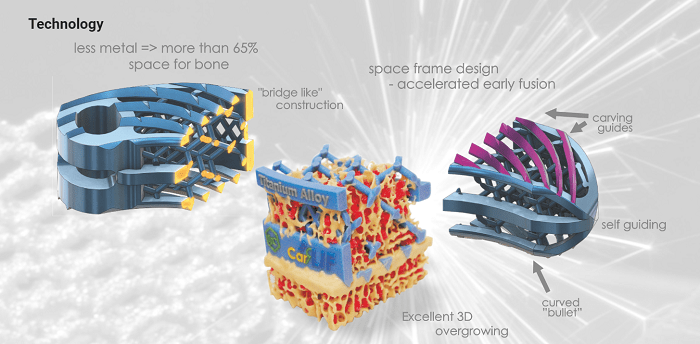
Benefits
- Uniquely shaped carving guides for:
- controlled insertion & precise placement;
- supreme primary stability;
- enlargement of load bearing surfaces;
- prevention against migration & expulsion;
- Anatomical „bullet” shape:
- smooth insertion;
- Specially designed 3D-Truss Ti structure:
- minimal volume of titanium;
- more than 65% space for bone ingrowth;
- light & strong construction;
- high load bearing capacity;
- surfaces of 3D-Truss elements susceptible to osteointegration;
- superior bony fusion
About LfC
LfC is a polish company who have achieved a leading position in the design and manufacture of surgical equipment used in spinal treatment in orthopaedics and neurosurgery.In the years 2007-2008 the company was honoured with prestigious awards, including the title of the Most Innovative Product in Poland (First Prize) in 2007 and the Golden Euro award in 2008. It also gained a high position among Polish innovative companies.Their product range includes Rod-Screws system, Anterior Cervical Plates, Posterior Cervical, Peek Cages, VBR implants and Titanium cages. http://www.lfc.com.pl/en/
