The Genesys Spine Binary® Lumbar Plate System provides an innovative solution to address instability of the spine from T1 to S1 caused by fractures, tumors, DDD, scoliosis, kyphosis, lordosis, spinal stenosis, or a failed previous spine surgery.
- Consists of Titanium plates with integrated locks and Titanium screws.
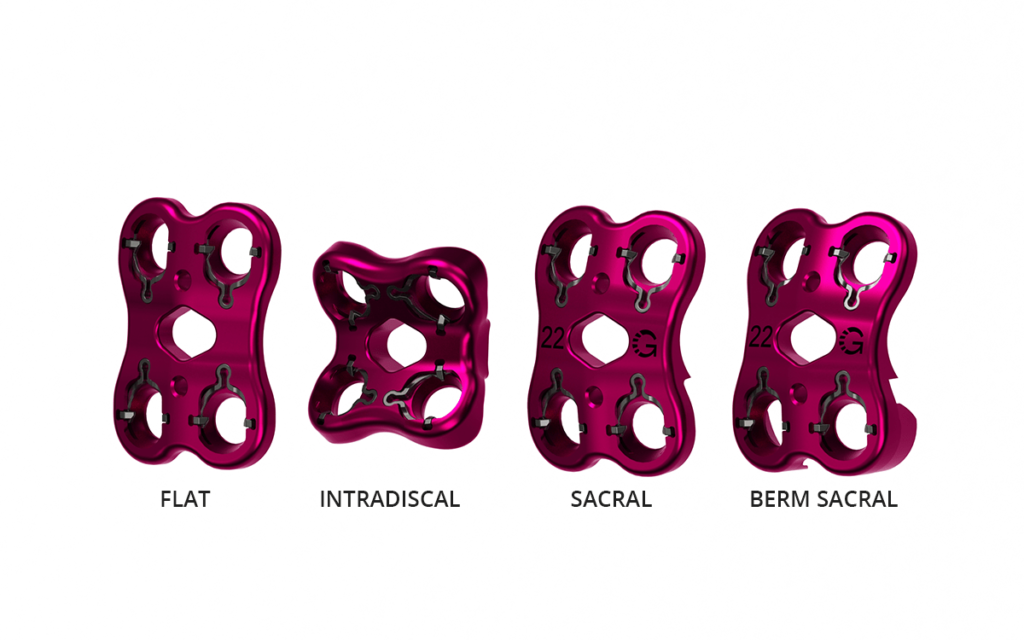
- One-Level (Flat) Plates, Intradiscal Plates, Sacral Plates, and Berm Sacral Plate options are available allowing for more configurable, patient specific fixation.
- Patented, award-winning Helios® Locking Mechanism prevents counter rotation and backout with audible, tactile, and visual feedback.
- Screws come in 2 diameters, 2 configurations (fixed and variable), with multiple lengths.
Features and Benefits
- The Lumbar Plates and Genesys interbodies can be loaded on the same Inserter for placement together.
- Simultaneous insertion of the plate and interbody can reduce the amount of steps, thus reducing OR time.
- Inserter can be used to deliver interbody and plate for lateral, anterolateral, or anterior surgical approaches.
- The same instruments are used for all three surgical approaches.
- Fixation Pins are available upon request.
About Genesys Spine
Founded in December of 2009, Genesys Spine’s mission was to bring a suite of medical implants and instruments with novel characteristics into a mature spinal fusion market. Since that time, Genesys has released eight new FDA-cleared product lines with several other products in various stages of the design and development cycle. Genesys has also developed several proprietary instruments to aid in the use of its products. All of Genesys’ inaugural products and proprietary features, including system-specific instruments, have garnered very positive feedback from the marketplace and remain within existing parameters for today’s current reimbursement codes. Find out more by visiting www.genesysspine.com
