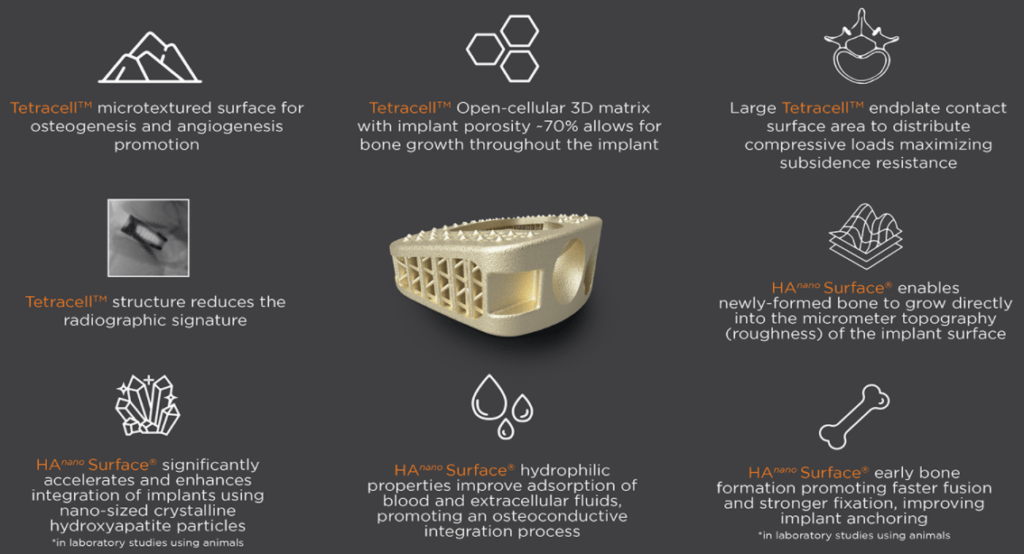
The AxTiHA™ System with Tetracell™ structure is a titanium standalone IBF device for Anterior Lumbar Interbody Fusion (ALIF) with up to 66% porosity1. The AxTiHA implant is integrated with HAnano Surface®, adding
enhanced fusion-friendly qualities to biocompatible features inherently found with implants made from titanium.
About Innovasis
Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and related products. Innovasis offers a spinal product line with implants and instruments that address the major pathologies and focus areas of traditional spinal surgery. Major spinal pathologies include; deformities, degenerative conditions, trauma and tumors, all of which can result in severe back pain and sometimes paralysis. These can occur in all areas of the spine, from the cervical region down through the thoracic, lumbar and sacral regions. Spinal implants are aimed at restoring mechanical and neurological function by readjusting vertebral positioning until bone fusion occurs. Innovasis is fully committed to providing surgeons and distributors with training, support and excellent customer service, thus ensuring the establishment of a strong and long term strategic partnership. http://www.innovasis.com
