CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended March 31, 2023, and recent corporate highlights.
Recent Highlights
- Extended momentum of PTPTM (Prone TransPsoas) procedure, the strongest contributor to Q1 revenue growth;
- Advanced lateral sophistication with full commercial release of LTPTM (Lateral TransPsoas) procedure;
- Introduced ATEC AIS (Adolescent Idiopathic Scoliosis) Approach, with adaptable InVictusTM instrumentation designed to streamline and optimize de-rotation;
- Drove 40% increase in surgical volume and 11% increase in average revenue per procedure;
- Acquired navigation-enabled robotics platform to enhance precision of ATEC’s procedural strategy.
“We continue to execute against our mission to revolutionize the approach to spine surgery,” said Pat Miles, Chairman and Chief Executive Officer. “As many in our industry capitulate, or seek to drive growth through acquired revenues, ATEC is content to be the outlier: methodically applying our 100% spine focus and unmatched know-how to integrate and evolve technologies that improve the predictability and reproducibility of spine care. ATEC’s innovation is not only driving rapid adoption today, it will also set new clinical standards for years to come.”
Financial Outlook for the Full-Year 2023
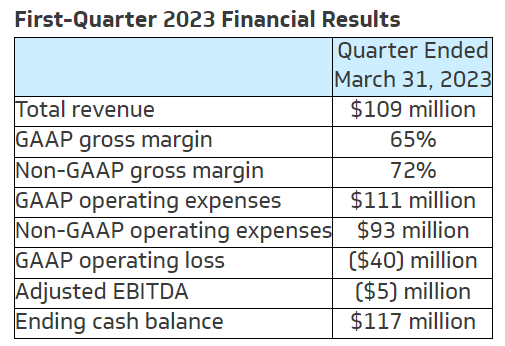
The Company continues to expect total revenue to grow 28% to $450 million for the fiscal year ended December 31, 2023, in line with expectations shared in conjunction with the release of preliminary first-quarter financial results. This includes surgical revenue growth of approximately 30% and $57 million of EOS revenue. The Company continues to expect to achieve non-GAAP adjusted EBITDA break-even for the full-year 2023.
Financial Results Webcast
ATEC will present these results via a live webcast today at 1:30 p.m. PT / 4:30 p.m. ET. The live webcast can be accessed by visiting the Investor Relations Section of ATEC’s Corporate Website.
To dial in to the webcast, please register via this link.
A replay of the webcast will remain available through the Investor Relations Section of ATEC’s Corporate Website for twelve months. In addition, a dial-in replay will be available beginning about two hours after the webcast’s completion through May 11, 2022. Access the replay by dialing (800) 770-2030 and referencing conference ID number 97241.
Non-GAAP Financial Information
To supplement the Company’s financial statements presented in accordance with generally accepted accounting principles in the United States of America (GAAP), the Company reports certain non-GAAP financial measures, including non-GAAP gross margin, non-GAAP operating expenses, non-GAAP operating loss, and non-GAAP adjusted EBITDA. The Company believes that these non-GAAP financial measures provide investors with an additional tool for evaluating the Company’s core performance, which management uses in its own evaluation of continuing operating performance, and a baseline for assessing the future earnings potential of the Company. The Company’s non-GAAP financial measures may not provide information that is directly comparable to that provided by other companies in the Company’s industry, as other companies in the industry may calculate non-GAAP financial results differently, particularly related to non-recurring, unusual items. Non-GAAP financial results should be considered in addition to, and not as a substitute for, or superior to, financial measures calculated in accordance with GAAP. Included below are reconciliations of the non-GAAP financial measures to the comparable GAAP financial measures.
About Alphatec Holdings, Inc.
ATEC, through its wholly owned subsidiaries, Alphatec Spine, Inc., EOS imaging S.A. and SafeOp Surgical, Inc., is a medical device company dedicated to revolutionizing the approach to spine surgery through clinical distinction. ATEC’s Organic Innovation MachineTM is focused on developing new approaches that integrate seamlessly with the Company’s expanding AlphaInformatiX Platform to better inform surgery and more safely and reproducibly achieve the goals of spine surgery. ATEC’s vision is to be the Standard Bearer in Spine. For more information, visit us at www.atecspine.com.
Forward Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainty. Such statements are based on management’s current expectations and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The Company cautions investors that there can be no assurance that actual results will not differ materially from those projected or suggested in such forward-looking statements as a result of various factors. Forward-looking statements include, but are not limited to: references to the Company’s revenue, balance sheet, growth and financial outlook; planned product launches and introductions; and the Company’s ability to compel surgeon adoption. Important factors that could cause actual operating results to differ significantly from those expressed or implied by such forward-looking statements include, but are not limited to: the uncertainty of success in developing new products or products currently in the pipeline; the uncertainties in the Company’s ability to execute upon its strategic operating plan; the uncertainties regarding the ability to successfully license or acquire new products, and the commercial success of such products; failure to achieve acceptance of the Company’s products by the surgeon community; failure to obtain FDA or other regulatory clearance or approval or unexpected or prolonged delays in the process; continuation of favorable first-party reimbursement; unanticipated expenses or liabilities or other adverse events affecting cash flow or the Company’s ability to achieve profitability; uncertainty of additional funding; product liability exposure; an unsuccessful outcome in any litigation; patent infringement claims; claims related to the Company’s intellectual property; and the Company’s ability to meet its financial obligations. A further list and description of these and other factors, risks and uncertainties can be found in the Company’s most recent annual report, and any subsequent quarterly and current reports, filed with the Securities and Exchange Commission. ATEC disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, unless required by law.
