According to reportsweb.com, the global Cervical Interbody Fusion Cages market will reach 1120 million US$ by the end of 2025, growing at a CAGR of 4.0% during 2018-2025. In recent years, the trend in the cervical spine market has been the use of stand-alone cages as it offers numerous advantages. On the other hand, the development of 3d Print techniques have made it possible to design porous titanium cages that increase the fusion rates.
1.-CoRoent Small Interlock™ | Nuvasive
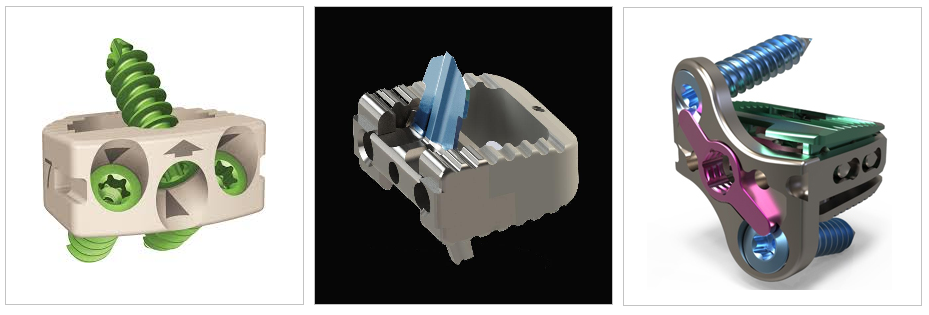
On Nov. 21st 2019, NuVasive announced the Company received U.S. Food and Drug Administration (FDA) 510(k) clearance for expanded indications for the CoRoent Small Interlock™ system. The expanded indication allows for on-label use of the device at multiple contiguous levels from C2-T1 for anterior cervical discectomy and fusion (ACDF) procedures, compared to other systems on the market cleared for only one- or two-level use.
The CoRoent Small Interlock system is an anterior cervical interbody fusion system indicated for use in adult patients with cervical disc degeneration and/or cervical spinal instability. The system is a no-profile interfixated device that is implanted entirely within the confines of the vertebral disc space and features a zero-step locking mechanism, a large fusion aperture, angled instrumentation for implantation in difficult anatomical locations and a three-screw implant design allowing for versatile interbody placement.
2.-TRIDENT | Pantheon Surgical
Pantheon’s ultimate goal is to produce successful patient outcomes utilizing minimally invasive, modular, and expandable implants and approaches. Pantheon is committed to changing healthcare, diminishing hospital overheard expenses, and decreasing payer cost.
Pantheon Surgical’s other products include the Pontus implant, the Epiphany implant, the Summit Spine Channel and Yellowstone Interbody fusion systems.
The TRIDENT Anchored Cervical Interbody Fusion single-step technology features a zero profile construct, titanium implant with PEEK radiolucent spacer, ample space for autogenous bone graft and a retractable anchor for easy implant removal.
3.-HiJAK SA | Atlas Spine
On March 10th, 2020 Atlas Spine Inc., announced clearance from the Food and Drug Administration for their HiJAK SA Expandable Stand-Alone Cervical Interbody System.
The HiJAK SA device leverages the design and clinical success of Atlas’s HiJAK AC Expandable Interbody platform, the market’s only cervical implant that provides surgeons the intra-operative ability to customize height and lordosis specific to their patient’s anatomy and surgical needs.
Product Animations in the Anterior Cervical Section:https://www.youtube.com/channel
Along with its expandable capability, HiJAK SA incorporates an integrated low-profile plate that significantly improves screw access during minimally invasive applications and screw-to-bone integrity, something current stand-alone devices struggle with.

4.- HEDRON IC™ 3D Printed ACDF Spacer | Globus Spine
On May 07, 2020, Globus Medical announced its financial results for the first quarter ended March 31, 2020 and also the launch of HEDRON line of 3D printed interbody spacers
HEDRON IC™ is a 3D printed ACDF spacer that can be used as an interbody spacer itself or combined with the COALITION AGX Plate and screws as a stand-alone plate-spacer. HEDRON IC™ features a biomimetic porous scaffolding designed to promote bone formation onto and through the implant.
Benefits:
- The Face of Fusion: An ovine interbody study demonstrated significantly more bone ingrowth within HEDRON™ implants at 6 weeks post-op compared to PEEK and solid titanium implants.*
- Intraoperative Integration: The HEDRON IC™ spacer and COALITION AGX® plate may be intraoperatively assembled for a low-profile approach requiring minimal retraction.
- Natural Anatomical Profile: The HEDRON IC™ Plate-Spacer is designed to preserve the natural anatomical profile of the cervical spine.
5.-Cervical Spine Truss System-Stand Alone | 4WEB Medical
On Aug. 28th , 2019, 4WEB Medical, announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Cervical Spine Truss System-Stand Alone (CSTS-SA) Interbody Fusion Device.
Consistent with 4WEB’s existing product portfolio, CSTS-SA has an open architecture, Advanced Structural Design that incorporates the company’s proprietary Truss Implant Technology™. The CSTS-SA product is designed to allow fixation screws to be placed through the truss implant and into the adjacent vertebral bodies creating a zero-profile stand-alone construct that removes the need for traditional plate and screw fixation.
Additionally, the device features a single-step locking mechanism that provides surgeon users confidence in the performance of the stand-alone construct. The Cervical Stand-Alone product line will be available in multiple footprints, lordotic angles, heights and will be delivered in sterile packaging for hospital efficiency and patient safety. 4WEB expects to take the product to market at the beginning of the fourth quarter.
4WEB Medical’s proprietary Truss Implant Technology™ utilizes the concept of mechanobiology to simulate cellular activity at the surgical site. The manufacturing process used to produce the implant’s Advanced Structural Design creates a hierarchical surface roughness with features that span from the macro to nano scale. In-vitro testing has shown that stem cells attached to 4WEB’s titanium implant surface exhibited increased gene expression of certain osteogenic markers when compared to smooth titanium and PEEK.
6.–F3D-C2 Stand-alone Cervical System | CoreLink Surgical
On June 30 th 2020 CoreLink announced the commercial launch and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the F3D-C2 Stand-alone Cervical System.
The system is comprised of an additively manufactured spacer with two bone screw anchors secured by a locking mechanism printed within the cage. Stand-alone cervical devices eliminate the need for a supplemental fixation plate, making anterior cervical discectomy and fusion (ACDF) procedures easier and faster to complete.
Patented and proven 3D printed Mimetic Metal® technology is incorporated into the spacer to emulate key characteristics of natural bone. The technology provides lower stiffness than machined titanium and the reduced implant density minimizes imaging artifact. Comprehensive instrumentation allows the device to be placed using freehand or guided techniques and modular guides facilitate working under a microscope.
