The Europe Spinal market is valued in USD 3000 Million in 2020. According to many studies, it will grow at a CAGR of 5,5% in the next four years.
Czech Republic and Poland as other countries in Europe have an increasing demand from the aging population, along with the introduction of 3D printed and custom fit spine implants. They are offering lucrative growth opportunities for the main companies as the demand for innovative products with high performance and efficiency is increasing. Cutting edge technology, engineering and medicine are combining to produce 3D titanium spinal implants.
1.-GLOBAL biomedica
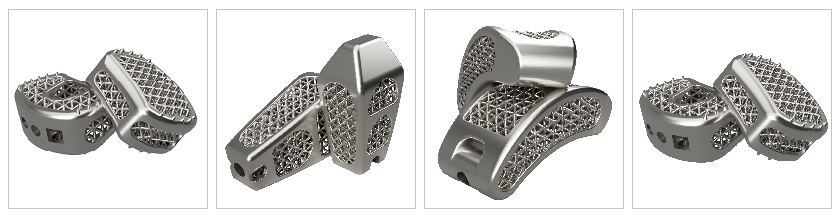
ISO 13485 certified, Czech Republic-based company specialize in producing interbody spinal fusion cages designed and constructed in collaboration with leading experts in this field, using innovative and high quality industrial 3D printing technologies.
All cages ( ACIF, PLIF, TLIF ) are recently CE marked and the company started to expand to European markets. Global Biomedica s.r.o. has invested in the latest technologies and follows the latest scientific knowledge, behind its main goal, to relieve patients, to minimize the risk of complications and to return patients to a happy and active life as quickly as possible. Internal and surface grid structure (Cube vertex centroid – lattice) with reinforced edges ensures not only high stability and resistance to deformation of the cage, or immersion in the vertebral body, but also bioactivity – potentiates the formation of bone in the area of contact surfaces and thus the formation of a strong connection between the cage and bone, the risk of developing nonunion ( pseudoarthrosis ) is thus minimized. This property is particularly outstanding when compared to the widely used PEEK implants (poly-ether-ether-ketone).
There is a wide range of products for ACIF, PLIF and TLIF fusion in many sizes. Thus, a wide range of modern tools for simple and, if necessary, minimally invasive cage insertion was designed. The latest 3D printing technology DMSL (Direct Metal Laser Sintering) is used in production.
Global Biomedica has invested in the latest technologies and follows the latest scientific knowledge, behind its main goal, to relieve patients, to minimize the risk of complications and to return patients to a happy and active life as quickly as possible. Global Biomedica s.r.o. is still moving forward and with prominent Czech and foreign spinal surgeons as well as with other specialists develops other spinal implants (ALIF, XLIF) and embarks on the most modern areas of medicine in individual cranial implants.
Company website: https://globalbmd.com/
Contact us: [email protected]
2.-SYNTROPIQ
SYNTROPIQ is a privately held spine interbody implant and surface technology company.Their team members with 15+ years in spine business, have seen over 10.000 of spine surgeries and have had hundreds of hours of discussions with spine surgeons.Headquartered in Prague (Czech Republic), they have recently launched the following 3D Cages:Aries (ACIF), Perseus (PLIF), Taurus (TLIF) and Ursus (ULIF).

Company website: http://www.syntropiq.com
3.-LfC
LfC is a polish company who have achieved a leading position in the design and manufacture of surgical equipment used in spinal treatment in orthopaedics and neurosurgery.In the years 2007-2008 the company was honoured with prestigious awards, including the title of the Most Innovative Product in Poland (First Prize) in 2007 and the Golden Euro award in 2008. It also gained a high position among Polish innovative companies.Their product range includes Rod-Screws system, Anterior Cervical Plates, Posterior Cervical, Peek Cages, VBR implants and Titanium cages. Some of their main products are the following:
Company website: http://www.lfc.com.pl/en/
