Vertebral Body Tethering is a non-fusion spinal procedure intended to treat idiopathic scoliosis, an abnormal curvature in the spine that occurs without a known cause, in young patients whose bones have not fully matured. The Vertebral Body Tethering System are made up of: anchors, bone screws, cord, and set screws. The anchors, bone screws and set screws are made out of titanium alloys that are commonly used as spine implants. The cord is made of a strong flexible polymer.
Which systems are available?
1.- The Tether™ – Vertebral Body Tethering System | Zimmer Biomet
Status: FDA’s approved. August 16, 2019
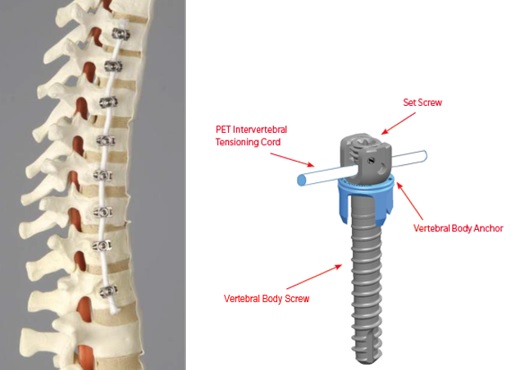
Zimmer Biomet’s anterior vertebral body tethering (AVBT) solution, The Tether, uses a strong, flexible cord made of SULENE® PET (polyethylene-terephthalate) , rather than metal rods, to pull on the outside of a scoliosis curve to initially straighten the spine, while the inside of the curve is left free to grow. This growth modulation approach now offers select, well-indicated patients an option to achieve a straighter spine, without the limitations of spinal fusion. Additionally, unlike fusion metallic rods, The Tether is positioned utilizing an endoscopic minimally invasive approach through a few small openings between the ribs.
How does it work? An anchor and bone screw are placed into the patient’s spine on the side of the spinal curvature. The cord is secured to the bone screws using set screws. During surgery, the surgeon will apply tension to the cord to partially straighten the patient’s spine. After surgery, the cord continues to straighten the spine while the patient continues to grow.
When is it used? The Tether™ – Vertebral Body Tethering System is indicated for skeletally immature patients that require surgical treatment to obtain and maintain correction of progressive idiopathic scoliosis, with a major Cobb angle of 30 to 65 degrees whose osseous structure is dimensionally adequate to accommodate screw fixation, as determined by radiographic imaging. Patients should have failed bracing and/or be intolerant to brace wear.
Advantages:
- Less invasive
- Less surgical hardware
- Curve reduction dependent on curve flexibility
- Less blood loss and risk of infection
- Can be used pre-skeletal maturity
- Spinal Motion sparing
- Long-term complications unknown
2.- REFLECT™ | Globus Medical
Status: Not FDA’s approved. Available Internationaly
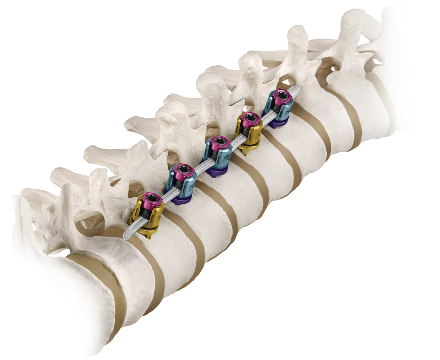
REFLECT™ is an anterior scoliosis correction system that is used to apply fixation on the convexity of the scoliotic vertebrae with a flexible cord to limit growth on the convex side and allow unilateral growth on the concave side.
Advantages:
- Minimally Invasive: Multi-functional instruments facilitate REFLECT™ cord insertion and tensioning using a mini-open anterior or posterior approach. REFLECT™ is designed to be implanted through a thorascopic approach to help minimize tissue disruption and scar tissue formation.
- Optimized Implant Offering: Low profile screws with a non-threaded locking mechanism and diameter-specific staples, used in conjunction with the REFLECT™ cord, create an optimized construct for spinal alignment to maintain stability while allowing growth in skeletally immature patients.
- Intraoperative Deformity Correction: Tensioning instruments allow for controlled curve correction with exceptional ease of use.
